Product Name: BIBF-1120
Synonyms: Nintedanib Esylate(BIBF-1120);Nintedanib, >=98%;Trinidad Neeb;Intedanib(BIBF-1120);BIBF1120 (VARGATEF|Nintedanib);BIBF1120 nintedanib;BIBF-1120;BIBF-1120(Vargatef)
CAS: 928326-83-4
MF: C31H33N5O4
MW: 539.633
EINECS:
Product Categories: -
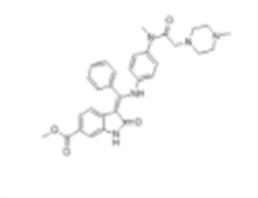
Mol File: 928326-83-4.mol
BIBF-1120 Structure
BIBF-1120 Chemical Properties
Safety Information
MSDS Information
BIBF-1120 Usage And Synthesis
Description BIBF 1120 is a triple kinase inhibitor blocking vascular endothelial growth factor receptor, PDGF receptor, and FGF receptor, which has a potential to inhibit tumor growth and pulmonary fibrosis.
Anticancer Research BIBF 1120 is a potent inhibitor of VEGFR as well as PDGF and fibroblast growth factor receptor. In a randomized phase II placebo-controlled trial, patients who had just completed chemotherapy for relapsed ovarian cancer, with evidence of response, but at high risk of further early recurrence were treated with BIBF 1120. The study drug was taken continuously (28-day cycles) for 9 cycles (36 weeks) or until disease progression or patient withdrawal. The 36-week PFS 26-week rates were 16.3% and 5.0% in the BIBF 1120 and placebo groups, respectively (HR 0.65; 95% Cl, 0.42 to 1.02; P = .06). Toxicity was also well tolerated.This has prompted a phase III trial (NCTO1O15118) where BIBF 1120 will be combined with carboplatin/paclitaxel as front-line chemotherapy in ovarian cancer.
Side effects Most of the adverse effects associated with BTBF-1120 were tolerable. They include diarrhea, nausea, vomiting, abdominal pain, and reversible increase in liver enzymes.
Clinical claims and research BIBF 1120 is a triple tyrosine kinase inhibitor with efficacy on fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and on platelet-derived growth factor (PDGF).The activation of these pathways has been implicated in the pathogenesis of experimental fibrosis. In a 12-month phase II trial (the TOMORROW trial) patients who received 150 mg of BIBF 1120 twice daily had a trend toward reduction in the decline of FVC.In addition, there was an improved QOL and a reduction in acute exacerbations of IPF. These results led to a phase III trial. This was designed as two identical phase III studies called INPULSIS-1 and INPULSIS-2. The INPULSIS trials tested the effect on IPF disease progression over 52 weeks using 150 mg of twice daily nintedanib (formerly BIBF 1200) versus placebo. The inclusion criteria included patients diagnosed with idiopathic pulmonary fibrosis based on established criteria.In addition, the participants’ HRCT scans and biopsies, if available, were reviewed by a central radiologist or pathologist to confirm the diagnosis. Enrolled subjects had a FVC which was >50% of the predicted value and a DlCO from 30–79% of predicted values. Published data from the two trials indicated that patients who received nintedanib demonstrated a statistically significant reduction in the rate of decline in lung function compared with the placebo group. In the INPULSIS-2 trial, there was a significant reduction in the time to first acute exacerbation of IPF. This was not replicated in the INPULSIS-1 trial data. The most common side effect for the medication was diarrhea. On the basis of this study, nintedanib is pending evaluation for approval for use in the United States.

 China
China