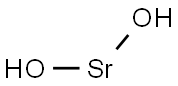Uses
Strontium hydroxide is used in extracting sugar from beet sugar molasses and in making lubricant soaps and greases.
Toxicity
Dry compound or aqueous solution is corrosive. Contact with skin or eyes can cause irritation.
Description
Strontium Hydroxide occurs as an anhydrate whose prismatic, colorless crystals are deliquescent. A monohydrate and octahydrate are also known.Strontium hydroxide is slightly soluble in water but, surprisingly, the octahydrate is much more soluble. It is the latter that is supplied commercially for most applications.When the octahydrate is heated, seven waters of hydration are lost to form the monohydrate and finally the oxide.
Chemical Properties
colourless deliquescent crystals
Uses
Extraction of sugar from beet sugar molasses, lubricant soaps and greases, stabilizer for plastics, glass, adhesives.
Uses
Strontium hydroxide [Sr(OH)2] is used to extract sugar from sugar beet molasses. It is also used in the manufacture of soaps, adhesives, plastics, glass, and lubricants that can be used in very high or low temperature environments.
Uses
Strontium hydroxide is used chiefly in the refining of beet sugar and as a stabilizer in plastic. It may be used as a source of strontium ions when the Cl- ion is undesirable. Strontium hydroxide absorbs carbon dioxide from the air to form strontium carbonate. This salt is available commercially in sizable quantities.
Purification Methods
Crystallise the hydroxide from hot water (2.2mL/g) by cooling to 0o.

 China
China