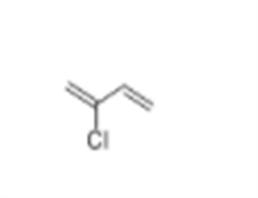
Product Name: Chloroprene
Synonyms: 2-chloro-1,3-butadiene(chloroprene);2-chloro-1,3-butadiene(chloroprene)(50%inxylene);2-chloro-3-butadiene;2-chloro-buta-1,3-diene;2-Chlorobuta-1,3-diene;2-Chlorobutadiene;2-Chlorobutadiene 1,3;2-chlorobutadiene-1,3
CAS: 126-99-8
MF: C4H5Cl
MW: 88.54
EINECS: 204-818-0
Product Categories: monomer
Mol File: 126-99-8.mol
Chloroprene Structure
Chloroprene Chemical Properties
Melting point -130°C
Boiling point 59.45°C
density 0,958 g/cm3
vapor pressure 118 at 10 °C, 200 at 20 °C, 275 at 30 °C (quoted, Verschueren, 1983)
refractive index 1.4583
Fp 11 °C
storage temp. 2-8°C
solubility Soluble in acetone, benzene, and ether (Weast, 1986)
Henry's Law Constant 3.20 x 10-2 atm?m3/mol using method of Hine and Mookerjee (1975)
Exposure limits Potential occupational carcinogen. NIOSH REL: 15-min ceiling 1 ppm (3.6 mg/m3), IDLH 300 ppm; OSHA PEL: TWA 25 ppm (90 mg/m3); ACGIH TLV: TWA 10 ppm (adopted).
CAS DataBase Reference 126-99-8(CAS DataBase Reference)
EPA Substance Registry System Chloroprene (126-99-8)
Safety Information
Hazard Codes F,T
Risk Statements 10-20/22-36-48/20-36/37/38-11-45-39/23/24/25-23/24/25
Safety Statements 16-45-53-36/37-7
RIDADR 1993
WGK Germany 3
HazardClass 3.1
PackingGroup I
Toxicity Acute oral LD50 for mice 260 mg/kg, rats 900 mg/kg (quoted, RTECS, 1985).
MSDS Information
Chloroprene Usage And Synthesis
Chemical Properties Chloroprene is a colorless, flammable liquid possessing a pungent odor. The Odor Threshold is 0.4 milligram per cubic meter
Physical properties Clear, colorless liquid with a pungent, ether-like odor. The odor threshold is 0.40 mg/m3 (CHRIS, 1984).
Uses Manufacture of neoprene.
Uses The only commercial use identified for chloroprene is as a monomer in the production of the elastomer polychloroprene (neoprene), a synthetic rubber used in the production of automotive and mechanical rubber goods, adhesives, caulks, flame-resistant cushioning,construction materials, fabric coatings, fiber binding, and footwear. Other uses of this polymer include applications requiring chemical, oil, or weather resistance or high gum strength. The U.S. Food and Drug Administration permits the use of chloroprene as a component of adhesives used in food packaging and also permits the use of polychloroprene in products intended for use with food (IARC 1979, 1999, NTP 1998).
Definition A colorless liquid derivative of butadiene used in the manufacture of neoprene rubber.
General Description A clear colorless liquid. Flash point -4°F. May polymerize exothermically if heated or contaminated. If polymerization takes place inside a container, the container may rupture violently. Less dense than water. Vapors heavier than air. Used to make neoprene rubber.
Air & Water Reactions Highly flammable. Slightly soluble in water.
Reactivity Profile CHLOROPRENE emits highly toxic fumes of chlorine gas when heated to decomposition. Autooxidizes very rapidly with air and, even at 0°C, produces unstable peroxides that catalyze exothermic polymerization [Bretherick, 5th ed., 1995, p. 507]. This reactivity is greatly slowed by presence of an inhibitor.
Hazard Toxic by ingestion, inhalation, and skin absorption. Flammable, dangerous fire risk, explosive limits in air 4.0–20%. Eye and upper respiratory tract irritant. Possible carcinogen.
Health Hazard INHALATION: Fatigue, psychic changes, irritability, oppression in chest, occasionally substernal pain, tachycardia upon exertion. EYES: Can cause conjunctivitis, corneal necrosis and edema of eyelids. SKIN: May cause dermatitis and temporary loss of hair. Rapidly absorbed by skin.
Safety Profile Confirmed carcinogen. Poison by ingestion, intravenous, and subcutaneous routes. Moderately toxic by inhalation. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. Human exposure has caused dermatitis, conjunctivitis, corneal necrosis, anemia, temporary loss of hair, nervousness, and irritabhty. Exposure to the vapor can cause respiratory tract irritation leading to asphyxia. Other effects are central nervous system depression, drop in blood pressure, severe degenerative changes in the liver, kidneys, lungs, and other vital organs. A very dangerous fire hazard when exposed to heat or flame. Explosive in the form of vapor when exposed to heat or flame. To fight fire, use alcohol foam. Auto-oxidlzes in air to form an unstable peroxide that catalyzes exothermic polymerization of the monomer. Incompatible with liquid or gaseous fluorine. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, ALIPHATIC .
Potential Exposure The major use of chloroprene is in the production of artificial rubber (Neoprene, duprene); polychloroprene elastomers. Chloroprene is extremely reactive, e.g., it can polymerize spontaneously at room temperatures; the process being catalyzed by light, peroxides, and other free radical initiators. It can also react with oxygen to form polymeric peroxides and because of its instability, flammability, and toxicity, chloroprene has no end-product uses as such.
Carcinogenicity Chloroprene is reasonably anticipated to be a human carcinogen based on evidence of carcinogenicity from studies in experimental animals.
Environmental fate Chemical/Physical. Anticipated products from the reaction of chloroprene with ozone or OH radicals in the atmosphere are formaldehyde, 2-chloroacrolein, OHCCHO, ClCOCHO, H2CCHCClO, chlorohydroxy acids, and aldehydes (Cupitt, 1980).
Chloroprene will polymerize at room temperature unless inhibited with antioxidants (NIOSH, 1997). Chloroprene is resistant to hydrolysis under neutral and alkaline conditions (Carothers et al., 1931).
Chloroprene is subject to hydrolysis forming 3-hydroxypropene and HCl. The reported hydrolysis half-life at 25 °C and pH 7 is 40 yr (Kollig, 1993).
Shipping UN1991 Chloroprene, stabilized, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous material.
Incompatibilities Can form unstable peroxides; chloroprene may polymerize on standing with fire or explosion hazard. May form explosive mixture with air. Reacts with liquid or gaseous fluorine, alkali metals; metal powders, oxidizers, creating a fire or explosion hazard. Attacks some plastics, rubber, and coatings. May accumulate static electrical charges, and may cause ignition of its vapors.
Waste Disposal Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.

 China
China