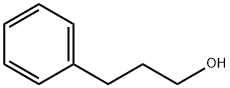
Chemical properties
Colorless viscous liquid with a sweet scent of flowers and sweetmeat and the flavor of fresh fruit after dilution. Its boiling point is at 236 °C, flash point at 109 °C. Soluble in ethanol, propylene glycol and most of the non-volatile oil, insoluble in glycerol and mineral oil, slightly soluble in water (1: 300).
Natural 3-Phenyl-1-propanol can be found in strawberries, Styrax cream, benzoin cream, tea, Peru Balsam, Cassia leaf oil, cinnamon oil and so on.
Uses
(1) Temporarily allowed as food flavouring agent in GB 2760-1996. Mainly used in the preparation of essence of peach, apricot, plum, watermelon, strawberry and nuts like walnut and hazel.
(2) Mainly used in the preparation of essence and medicine.
An intermediate of proformiphen (a central skeletal muscle relaxant)
Temporarily allowed as food flavouring agent in GB 2760-1996.
It has a sweet scent of flowers and sweetmeat and a pleasant flavor of fresh fruit after dilution
Natural 3-Phenyl-1-propanol can be found in strawberries, Styrax cream, benzoin cream, tea, Peru Balsam, Cassia leaf oil, cinnamon oil and so on.
(3) As a medicine, it can be applied to Cholecystitis, cholangitis, cholelithiasis, biliary postoperative syndrome, hypercholesterolemia, etc.
(4) Mainly used in the preparation of essence and medicine.
An intermediate of proformiphen, a central skeletal muscle relaxant.
As an antiputrefactiva in cosmetic in combination with piperonal or pepper, 3-Phenyl-1-propanol has a mold resistance on germ and mycete, along with a natural fragrance.
3-Phenyl-1-propanol or its ester derivatives can be used as flavor constituents of flowers such as lilac, hyacinth and keiskei for its aromatic vinegar.
content analysis
Total alcohol method (OT-5)Sample amount : l g, Equivalent Factor (f) = 68.10.
nonpolar column method of gas chromatography (GT-10-4)
toxicity
GRAS(FEMA).
LD502300mg/kg (Rats oral).
Security utilization limitation
FEMA(mg/kg):0.73 in soft drinks; 1.4 in cold drink; 2.8 in sweets; 3.3 in baked food; 4.3 in gum confection; 5.0 in liquor. WHO Class II /moderate toxicity
Moderate limit (FDA§172.515,2000).
Production Method
(1) the catalytic hydrogenation of ethyl cinnamate.
The hydrogenation reaction is conducted in autoclave with chromium-copper-barium catalyst at 200℃ and 20MPa for 5-9h. The filtrate obtained after cooling and filtration is extracted by diethyl ether. After the recycling of diethyl ether, reduced pressure distillation of the extracting solution is conducted to collect the fraction of 110-112℃(1.06kPa), which is the finished product. The yield is about 85%.
Grignard reaction of benzyl chloride and oxirane, followed by the hydrolysis with sulfuric acid to obtain 3-Phenyl-1-propanol. The yield is about 65-70%。
(2) The hydrogenation of peruvin or cinnamaldehyde.
Standard for Maximum Allowable Amount
food additives: phenylpropanol
allowable usage:food
function of additives:Food flavouring
maximum allowable amount of usage (g/kg): The essence ingredients used in shall not exceed the maximum permissible usage and residues allowed in GB 2760.
maximum allowable amount of residue(g/kg): The essence ingredients used in shall not exceed the maximum permissible usage and residues allowed in GB 2760.
Chemical Properties
CLEAR COLOURLESS LIQUID
Chemical Properties
3-Phenyl-1-propanol occurs both in free and esterified forms in resins and balsams (e.g., benzoe resin and Peru balsam). It has been identified in fruits and cinnamon.
Hydrocinnamic alcohol is a slightly viscous, colorless liquid with a floralbalsamic odor, slightly reminiscent of hyacinths. Esterification with aliphatic carboxylic acids is important because it leads to additional fragrance and flavor materials.
Hydrocinnamic alcohol is prepared by hydrogenation of cinnamaldehyde. A mixture of hydrocinnamic alcohol and the isomeric 2-phenylpropanol can be obtained from styrene by a modified oxo synthesis. The two isomers can be separated by distillation.
Hydrocinnamic alcohol is used in blossom compositions for balsamic and oriental notes.
Chemical Properties
3-Phenyl-1-propanal has a characteristic sweet, hyacinth-mignonette odor. It has a sweet and pungent taste suggestive of apricot.
Occurrence
Reported found in storax, Sumatra benzoin, tea, Peru balsam, passion fruit, strawberry, bilberry, high bush blueberry, European cranberry, guava peel, fresh blackberry, heated blackberry, rum, white wine, shitake, matsutake, peated malt, loquat, sapodilla fruit and crownberry.
Preparation
By hydrogenation of either cinnamic aldehyde or cinnamic alcohol.
Taste threshold values
Taste characteristics at 20 ppm: spicy, cinnamon, balsamic, fruity, winy and honey-like with floral nuances.

 China
China