Description
Asenapine, a dual antagonist of dopamine D2and serotonin 5-HT2receptors, was launched for the acute treatment of schizophreniaand manic or mixed episodes associated with bipolar I disorder in adults. Asenapine exhibits high affinity for serotonin 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5, 5-HT6, and 5-HT7 receptors (Ki = 2.5, 4.0, 0.06, 0.16, 0.03, 1.6, 0.25, and 0.13 nM, respectively); dopamine D1, D2, D3, and D4 receptors (Ki = 1.4, 0.42, 1.1, and 1.3 nM, respectively); a1- and a2-adrenergic receptors (Ki = 1.2 and 1.2 nM, respectively); and histamine H1 receptors (Ki = 1.0 nM), and moderate affinity for H2 receptors (Ki = 6.2 nM). In in vitro assays, asenapine acts as an antagonist at these receptors. Asenapine has no appreciable affinity for muscarinic cholinergic receptors (e.g., Ki = 8128 nM for M1). Despite its potent binding affinity for multiple receptors, the efficacy of asenapine in schizophrenia is thought to be primarily mediated through a combination of antagonist activity at D2 and 5-HT2A receptors.
Side effects
The most common adverse effects associated with asenapine treatment include insomnia, somnolence, nausea, anxiety, agitation, headache, vomiting, constipation, and psychosis. In placebo-controlled trials with risperidone, aripiprazole, and olanzapine in elderly subjects with dementia, there was a higher incidence of cerebrovascular adverse reactions (cerebrovascular accidents and transient ischemic attacks) including fatalities compared to placebo-treated subjects. Asenapine is not approved for the treatment of patients with dementia-related psychosis.
Chemical Synthesis
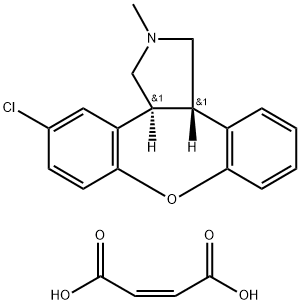
The chemical synthesis of asenapine can be achieved by several different methods. A concise synthesis of asenapine begins with the Horner-Emmons condensation of diethyl (5-chloro-2-fluoro)benzyl phosphonate with salicylaldehyde to produce the corresponding stilbene derivative, which undergoes a 1,3-dipolar cycloaddition reaction with trimethylamine-N-oxide in the presence of lithium diisopropylamide to furnish a trans-3,4-diarylpyrrolidine intermediate. Then, a base-catalyzed intramolecular cyclization involving the nucleophilic displacement of the fluoro group by the phenolic hydroxyl group affords asenapine, which is finally treated with maleic acid in ethanol to yield the corresponding maleate salt.

 China
China