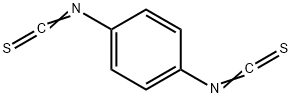
Chemical Properties
off-white to yellow to beige-grey crystalline
Chemical Properties
Those engaged in the manufacture, formulation and application of this anthelmintic compound. Incompatibilities: In general, keep away from strong oxidizers, moisture, strong acids, strong bases. This is a thiocyanate compound. Violent reactions may occur upon contact with chlorates (potassium chlorate, sodium chlorate), nitrates, nitric acid; organic peroxides.
Uses
Reagent for solid-phase sequencing.
General Description
Odorless colorless crystals. Melting point 132°C.
Reactivity Profile
1,4-PHENYLENE DIISOTHIOCYANATE reacts readily with amines [Noller]. These reactions may generate significant amounts of heat . Emits very toxic fumes of nitrogen oxides and sulfur oxides when heated to decomposition [EPA, 1998].
Health Hazard
1,4-PHENYLENE DIISOTHIOCYANATE is highly toxic if ingested. It is a central nervous system and gastrointestinal toxin in humans.
Fire Hazard
When heated to decomposition, 1,4-PHENYLENE DIISOTHIOCYANATE emits very toxic fumes of nitrogen oxides and sulfur oxides.
Safety Profile
Poison by ingestion and intraperitoneal routes. Human systemic effects by ingestion: hallucinations, nausea. When heated to decomposition it emits very toxic fumes of NOx,CN,and SOx.See also THIOCYANATES
Potential Exposure
Those engaged in the manufacture, formulation and application of this anthelmintic compound. Incompatibilities: In general, keep away from strong oxidizers, moisture, strong acids, strong bases. This is a thiocyanate compound. Violent reactions may occur upon contact with chlorates (potassium chlorate, sodium chlorate), nitrates, nitric acid; organic peroxides.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.
Purification Methods
Purify bitoscanate by recrystallisation from AcOH, pet ether (b 40-60o), Me2CO or aqueous Me2CO. [van der Kerk et al. Recl Trav Chim Pays-Bas 74 1262 1955, Leiber & Slutkin J Org Chem 27 2214 1962, Beilstein 13 IV 174.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.

 China
China