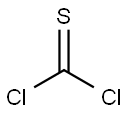
Chemical Properties
Reddish liquid. Decomposes in water and alcohol; soluble in ether.
Chemical Properties
A clear dark red to reddish-yellow liquid. Sharp, choking odor.
Uses
Thiophosgene is a photo degradation product of the agricultural fungicide Folpet (F402000).
General Description
A reddish liquid. Boiling point 73.5°C. A severe eye irritant. May severely burn skin on contact. Very toxic by inhalation and by skin absorption.
Air & Water Reactions
Reacts with water to evolve hydrogen chloride, carbon disulfide, and carbon dioxide. Reaction is slow unless the water is hot.
Reactivity Profile
Thiophosgene is incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Liberates hydrogen sulfide upon reaction with acids.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Inhalation causes irritation of respiratory system and delayed pulmonary edema. Vapor irritates eyes. Liquid burns skin and eyes. Ingestion causes irritation of mouth and stomach.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. A skin, mucous membrane, and severe eye irritant. When heated to decomposition it emits very toxic fumes of Cland SOx. See also PHOSGENE.
Potential Exposure
Primary irritant (w/o allergic reaction). There is not large-scale production of the chemical in the United States It is used to make other chemicals and in laboratory synthesis.
Shipping
UN2474 Thiophosgene, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard; Inhalation Hazard Zone B. PG 2. STN: 49 232 98.
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with water and alcohols. Reacts with water releasing toxic hydrogen chloride, carbon disulfide, and carbon dioxide. Reaction is slow unless the water is hot. Decomposes above 200℃ to highly flammable carbon bisulfide and carbon tetrachloride. Corrodes metals, rubber and some plastics in the presence of moisture. Thiophosgene is incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Liberates hydrogen sulfide upon reaction with acids
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.

 China
China