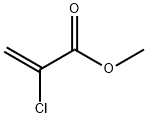
Chemical Properties
clear colorless to pale yellow liquid
Chemical Properties
Methyl 2-chloroacrylate is a colorless liquid.
General Description
Colorless liquid. Used to make acrylic high polymer with properties closely resembling those of polymethylmethacrylate. Monomer for specialty polymers (e.g., aircraft glazing).
Air & Water Reactions
Insoluble in water.
Reactivity Profile
METHYL ALPHA-CHLOROACRYLATE is sensitive to prolonged exposure to light, moisture and heat. METHYL ALPHA-CHLOROACRYLATE is incompatible with oxidizers and polymerizers.
Health Hazard
METHYL ALPHA-CHLOROACRYLATE is a skin, eye, and lung irritant. The least trace on skin raises large blisters. It is also a respiratory poison; breathing the vapors can cause pulmonary edema.
Fire Hazard
Flash point data for METHYL ALPHA-CHLOROACRYLATE are not available; however, METHYL ALPHA-CHLOROACRYLATE is probably combustible.
Potential Exposure
Used to make acrylic high polymer with properties closely resembling those of polymethylmethacrylate. Monomer for specialty polymers e.g., aircraft glazing). Corrosive. Lacrimator.
Shipping
UN2929 Toxic liquids, flammable, organic, n.o.s., Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, Technical Name Required.
Incompatibilities
apor may form explosive mixture with air. May hydrolyze upon contact with moisture. Incompatible with nitrates. Methyl 2-chloroacrylate is sensitive to prolonged exposure to light, moisture and heat. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and polymerizers. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). May be a polymerization hazard

 China
China