| Chemical Properties |
Colorless gas at room temperature; fishy ammoniacal odor; readily liquefied. Anhydrous form shipped as liquefied compressed gas. Soluble in water, alcohol, and ether. |
| Uses |
Organic synthesis, especially of choline salts, warning agent for natural gas, manufacture of disinfectants, flotation agent, insect attractant, quaternary ammonium compounds, plastics. |
| Definition |
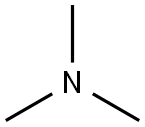
ChEBI: A tertiary amine that is ammonia in which each hydrogen atom is substituted by an methyl group. |
| General Description |
A colorless gas with a fishlike odor at low concentrations changing to ammonia-like odor at higher concentrations. Shipped as a liquid under its own vapor pressure. Contact with the unconfined liquid can cause frostbite from evaporative cooling or chemical type burns. The gasis corrosive and dissolves in water to form flammable, corrosive solutions. Gas is an asphyxiate by the displacement of air. Produces toxic oxides of nitrogen during combustion. Prolonged exposure to heat can cause the containers to rupture violently and rocket. Long-term inhalation of low concentrations or short -term inhalation of high concentrations has adverse health effects. |
| Air & Water Reactions |
Highly flammable and easily ignited. Water soluble. |
| Reactivity Profile |
TRIMETHYLAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. Contamination of an ethylene oxide tank with trimethylamine caused an explosion [BCISC Quart. Safety Summ., 1966, 37, 44]. |
| Health Hazard |
VAPOR: POISONOUS IF INHALED. Irritating to eyes, nose, and throat. LIQUID: Will burn skin and eyes. Harmful if swallowed. |
| Fire Hazard |
FLAMMABLE. Flashback along vapor trail may occur. Vapor may explode if ignited in an enclosed area. Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back. |
| Safety Profile |
Poison by intravenous route. Moderately toxic by subcutaneous and rectal routes. Mildly toxic by inhalation. A very dangerous fire hazard when exposed to heat or flame. Self-reactive. Moderately explosive in the form of vapor when exposed to heat or flame. Can react with oxidizing materials. To fight fire, stop flow of gas. Potentially explosive reaction with bromine + heat, ethylene oxide, triethynylaluminum. When heated to decomposition it emits toxic fumes of NOx. See also AMINES. |
| Purification Methods |
Dry triethylamine by passing the gas through a tower filled with solid KOH. Water and impurities containing labile hydrogen were removed by treatment with freshly sublimed, ground, P2O5. It has been refluxed with acetic anhydride, and then distilled through a tube packed with HgO and BaO. [Comyns J Chem Soc 1557 1955.] For more extensive purification, trimethylamine is converted to the hydrochloride, crystallised (see below), and regenerated by treating the hydrochloride with excess aqueous 50% KOH, the gas is passed through a CaSO4 column into a steel cylinder containing sodium ribbon. After 1-2 days, the cylinder is cooled to -78o and hydrogen and air are removed by pumping. [Day & Felsing J Am Chem Soc 72 1698 1950.] Me3N has been distlled from trap-to-trap and degassed by freeze-pump-thaw [Halpern et al. J Am Chem Soc 108 3907 1986]. It is commercially supplied in a pressure tin. [Beilstein 4 H 43, 4 I 322, 4 II 553, 4 III 99, 4 IV 134.]
|

 China
China