| Chemical Properties |
Yellow liquid.Discolors and stains badly. |
| Uses |
Antifungal |
| Uses |
Antioxidant in feed and food; antidegradation agent for rubber. |
| Definition |
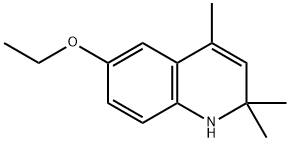
ChEBI: A quinoline that is 1,2-dihydroquinoline bearing three methyl substituents at position 2, 2 and 4 as well as an ethoxy substituent at position 6. |
| General Description |
Clear light yellow to dark brown viscous liquid. Discolors and stains badly. Mercaptan-like odor. |
| Air & Water Reactions |
Polymerizes and darkens in color on exposure to light and air. Insoluble in water. |
| Reactivity Profile |
Ethoxyquin may undergo a hazardous polymerization at temperatures above 320° F. Tends to polymerize and darken in color on exposure to light and air. Not compatible with oxidizing agents and with strong acids . |
| Hazard |
Toxic by ingestion. |
| Fire Hazard |
Ethoxyquin is combustible. |
| Agricultural Uses |
Insecticide, Fungicide, Plant growth regulator, Ingredient in other products: Used for preharvest or postharvest preservation of color in apples and pears. It is used as an antioxidant to preserve color in paprika and ground and powdered chili. Ethoxyquin is also a chemical preservative used in animal feed to prevent ingredients from reacting with oxygen and becoming rancid. It has been known to cause birth defects in pet birds and dogs. Not approved for use in EU countries[ 115]. Registered for use in the U.S. |
| Trade name |
CHEMLEY®[C]; DECCOQUIN 305®; EMQ®; EQ®; NIFLEX®; NIX-SCALD®[C]; SANTOFLEX A®; SANTOFLEX AW®; SANTOQUIN®; SANTOQUINE®; STOP-SCALD® |
| Contact allergens |
Ethoxyquin is used as an antioxidant in animal feed and caused contact dermatitis in a worker at an animal feed mill |
| Safety Profile |
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. When heated to decomposition it emits toxic fumes of NOx. |
| Purification Methods |
Purify Ethoxyquin by fractional distillation in vacuo whereby the distillate solidifies to a glass. [Knoevenagel Chem Ber 54 1723, 1730 1921.] The methiodide has m 179o (from EtOH), and the 1-phenylcarbamoyl derivative has m 146-147o (from EtOH). [Beaver et al. J Am Chem Soc 79 1236 1957, Beilstein 21 III/IV 95.] |

 China
China