| Chemical properties |
The pure triadimenol is white, tasteless fine crystalline powder. m.p.: 112 ° C, vapor pressure: 1 × 10 -3 Pa (20 ° C). Solubility in organic solvents: cyclohexane 40%, isopropanol 15%, methylene chloride 10%, toluene 4%; Solubility in water: 0.12g / L. Stable in neutral or weak acid medium, easy to break down when boiled in strong acidic medium. |
| Uses |
It is a systemic broad-spectrum seed treatment fungicide which can kill pathogens attached to the internal and external surface of seed. It works through the inhibition of fungal ergosterol biosynthesis. It’s used on cereal grains to control powdery mildew and smut diseases such as loose smut, stinking smut and root rot on wheat, loose smut, rust, leaf stripe disease and net blotch etc on barley, and it is more than 90% effective when 100kg of seeds are treated with 30 ~ 50g of 25% DS. It is more than 90% effective when 100kg of seeds are treated with 42 ~ 75g of 25% DS to control smut on corn and sorghum head smut. If 100kg of seeds are treated with 30g of triadimenol and 5g of furidazol, it will be 92% to 100% effective for the control of loose smut, stripe disease, net blotch and root rot on spring barley , loose smut, stinking smut, snow mold on winter wheat, and leaf stripe disease, loose smut on spring oat.
Triadimenol is a broad-spectrum fungicide with high efficiency and low toxicity. It is used on wheat and rice to control rust, powdery mildew, sheath blight and other diseases, and has a significant yield increasing effect. It can be also used as a cereal seed treatment and for the control of cereal smut. |
| Toxicity |
The acute oral toxicity LD50 in rats was 1161 mg / kg (female), 1105 mg / kg (male). The acute oral toxicity LD50 in mice was 1300 mg / kg (male & female). No effect dose for dogs and rats during 90 days feeding test: 600mg / kg • d. The acute inhalation The LC50 in goldfish and rainbow trout was 10~15mg/L and 23.5mg/L respectively (both 96 hours). The LD50 in quail was > 1000mg / kg. No effect on bees. |
| Preparation method |
Mostly, using triadimefon as the starting material , triadimenol is prepared through different reduction processes.
1. Reduction by hydrogen in the presence of a catalyst and any polar solvent, or reduction by aluminum isopropoxide in the presence of a solvent: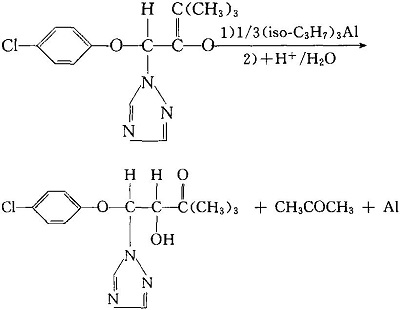
2. Reduction by borohydride in the presence of any polar solvent.
3. Reduction by formamidine sulfinic acid and alkali metal hydroxide in the presence of any polar solvent: 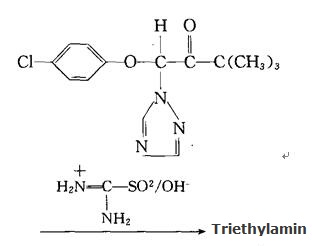
4. Reduction by using formic acid - triethylamine adduct as a reducing agent: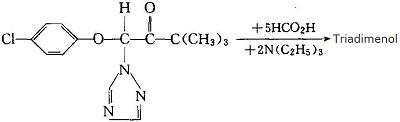 |
| Uses |
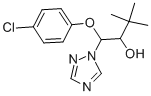
β-(4-Chlorophenoxy)-α-(1,1-dimethylethyl)-1H-1,2,4-triazole-1-ethanol is a agricultural fungicide that is systemically active against powdery mildews and rusts of grains. |
| Definition |
ChEBI: A member of the class of triazoles that is 3,3-dimethyl-1-(1,2,4-triazol-1-yl)butane-1,2-diol substituted at position O1 by a 4-chlorophenyl group. A fungicide for cereals, beet and brassicas used to control a range of diseases including powdery mildew, ru ts, bunts and smuts. |
| Uses |
Systemic agricultural fungicide; cereal seed protectant. |
| Agricultural Uses |
Fungicide: Triadimenol is used to control seed-and soil-borne diseases and to provide early season control of foliar diseases. It is applied to seeds of barley, corn, oats, rye, sorghum and wheat and also to fruits, vegetables and ornamentals. Registered for use in EU countries . Registered for use in the U.S. U.S. Maximum Allowable Residue Levels for Triadimenol |
| Trade name |
BAYFIDAN®; BAYFRDAN EW®; BAY KWG 0519®; BAYTAN® SEED TREATMENT; BAYTAN 30® FUNGICIDE; PROTEGE ALLEGIANCE BAYTAN®; SPINNAKER®; SUMMIT®; TRIADIMENOL®; TRIAFOL®; TRIAPHOL® |

 China
China