| Description |
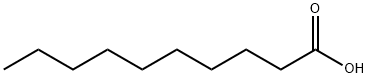
Decanoic acid (capric acid) is a saturated fatty acid with a 10-carbon backbone. It occurs naturally in coconut oils, palm kernel oil, and the milk of cow/goat.
Capric acid is most commonly used in the cosmetic and personal care, food/beverage, and pharmaceutical industries. It is also used as an intermediate in chemical syntheses. Furthermore, it is used in organic synthesis and in the manufacture of lubricants, greases, rubber, plastics, and dyes. |
| References |
[1] https://www.efsa.europa.eu
[2] https://circabc.europa.eu
[3] http://www.chemicalland21.com
[4] http://www.prnewswire.com/news-releases/global-capric-acid-market-2017-2021-300423638.html |
| Chemical Properties |
white crystals with an unpleasant odour |
| Uses |
Decanoic acid is used in manufacturing of esters for artificial fruit flavors and perfumes. |
| Uses |
Intermediates of Liquid Crystals |
| Definition |
ChEBI: A C10, straight-chain saturated fatty acid. |
| Uses |
manufacture of esters for artificial fruit flavors and perfumes; as an intermediate in other chemical syntheses. |
| General Description |
White crystalline solid with a rancid odor. Melting point 31.5°C. Soluble in most organic solvents and in dilute nitric acid; non-toxic. Used to make esters for perfumes and fruit flavors and as an intermediate for food-grade additives. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
Capric acid reacts exothermically to neutralize bases. Can react with active metals to form gaseous hydrogen and a metal salt. May absorb enough water from the air and dissolve sufficiently in Capric acid to corrode or dissolve iron, steel, and aluminum parts and containers. Reacts with cyanide salts or solutions of cyanide salts to generate gaseous hydrogen cyanide. Reacts exothermically with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides to generate flammable and/or toxic gases. Can react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reacts with carbonates and bicarbonates to generate a harmless gas (carbon dioxide). Can be oxidized exothermically by strong oxidizing agents and reduced by strong reducing agents; a wide variety of products is possible. May initiate polymerization reactions or catalyze (increase the rate of) reactions among other materials. |
| Health Hazard |
Harmful if swallowed or inhaled. Material is irritating to tissues of mucous membranes, and upper respiratory tract, eyes and skin. |
| Fire Hazard |
Capric acid is combustible. |
| Safety Profile |
Poison by intravenous route. Mutation data reported. A moderate skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Purification Methods |
The acid is best purified by conversion into its methyl ester, b 114.0o/15mm (using excess MeOH, in the presence of H2SO4). The H2SO4 and MeOH are removed, the ester is distilled in vacuo through a 3ft column packed with glass helices. The acid is then obtained from the ester by saponification and vacuum distillation. [Trachtman & Miller J Am Chem Soc 84 4828 1962, Beilstein 2 IV 1041.] |

 China
China