| Description |
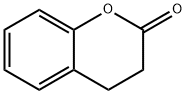
With a sweet, creamy, and herbal, fragrance, with a slightly burnt taste, dihydrocoumarin (DHC) is used as a flavoring agent in food, tobacco, soap, and perfume, etc. Its exotic flavor is well suited for caramel, nuts, dairy, vanilla, tropical fruit, and alcohol. It is a eukaryotic metabolite found in tonka beans grown in northern South America, from which it was isolated as early as the 1820s, as well as sweet clover. Other uses include as an organic solvent and pharmaceutical intermediary. It has been shown to influence the epigenetic process of human cells in vitro. |
| Sources |
http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:16151
http://www.bojensen.net/EssentialOilsEng/EssentialOils29/EssentialOils29.htm#Tonka
https://books.google.kg/books?id=pUEqBgAAQBAJ&pg=PA427&lpg=PA427&dq=dihydrocoumarin+uses&source=bl&ots=HTZrffvsXu&sig=GPGKqrMRXQaRJ-qgHk7aULeBmGw&hl=en&sa=X&redir_esc=y#v=onepage&q=dihydrocoumarin%20uses&f=false
https://products.symrise.com/aroma-molecules/product-search/dihydrocoumarin/action/pdf/
http://www.lookchem.com/3-4-Dihydrocoumarin/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1315280/ |
| Chemical Properties |
clear light yellow to brown liquid after melting |
| Definition |
ChEBI: A chromanone that is the 3,4-dihydro derivative of coumarin. |
| General Description |
White to pale yellow clear oily liquid with a sweet odor. Solidifies around room temperature. |
| Air & Water Reactions |
Solutions of the chemical in water are stable for less than two hours. Insoluble in water. |
| Reactivity Profile |
Hydrocoumarin is a lactone (behaves as an ester). Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Hydrocoumarin may hydrolyze under alkaline or acidic conditions. |
| Fire Hazard |
Hydrocoumarin is combustible. |

 China
China