| Overview |
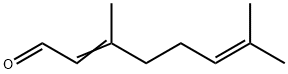
Citral (C10H16O), also called 3,7-dimethyl-2,6-octadienal, a pale yellow liquid, with a strong lemon odour, that occurs in the essential oils of plants. It is insoluble in water but soluble in ethanol (ethyl alcohol), diethyl ether, and mineral oil. It is used in perfumes and flavourings and in the manufacture of other chemicals. Chemically, citral is a mixture of two aldehydes that have the same molecular formula but different structures.
Content analysis
Accurately weigh about 1g of the sample, and then perform the determination by the hydroxylamine method (OT-7, method one) used in aldehyde and ketone determination. The equivalent factor (e) in the calculation is 76.12. |
| Toxicity |
ADI 0~0.5mg/kg (FAO/WHO, 1994-). LD50 4960 mg/kg (rat, oral); MNL 500 mg/kg. |
| Usage limits |
FEMA (mg/kg): soft drinks 9.2; cold drinks 23; candy 41; baked goods 43; chewing gums 170 |
| Chemical Properties |
Colorless or slightly yellow liquid; strong lemon flavor; no optical rotation; boiling point 228 °C; flash point 92 °C;
There are cis and trans two isomers. With sodium bisulfite treatment, cis isomer solubility is minimal, while the trans isomer solubility is very large, so the two isomers can be separated.
Cis citral: relative density (d20) 0.8898, refractive index (nD20) 1.4891, boiling point 118~119℃ (2666Pa).
Trans citral: relative density (d20) 0.8888, refractive index (nD20) 1.4891, boiling point 117~118℃ (2666Pa).
Soluble in non-volatile oils, volatile oils, propylene glycol and ethanol; insoluble in glycerol and water; unstable in alkaline and strong acids
Natural products present in lemon grass oil (70% to 80%), litsea cubeba oil (about 70%), lemon oil, white lemon oil, citrus leaf oil and so on. |
| Application |
Citral is an artificial flavor permitted to use in China, which can be used to prepare strawberries, apples, apricots, sweet orange, lemon and other fruit-based flavors. According to normal production needs, the citrals amount used in chewing gums is 1.70mg/kg; baked goods 43mg/kg; candy 41mg/kg; cold drinks 23mg/kg; soft drinks 9.2mg/kg.
It is also widely used in dishwashing detergents and the flavoring agents of soap and toilet water. Citral can be used as the raw material to synthesize ionone, methyl ionone and dihydro damascene. As organic raw material, it can also be reduced to generate citronellol, nerol alcohol and geraniol, and be converted into lemonile. In the pharmaceutical industry, it can be used for the manufacture of vitamin A and E, and also as the raw material of chlorophyll. |
| Production method |
Citral natural exists in the litsea cubeba oil (about 80%), lemon grass oil (80%), clove basil oil (65%), sour lemon oil (35%) and lemon oil. In industry, citral can be derived from natural essential oils, or be prepared by chemical.
Synthesis based on methyl heptenone as raw material
Ethoxyacetylene magnesium bromide and methyl heptenone performed condensation reaction to form 3,7-dimethyl-1-ethoxy-3-hydroxy-6-octene-1-yne, which was then partly hydrogenated in the presence of catalysis to generate enol ether. And the enol ether was then hydrolyzed with phosphoric acid and dehydrated to obtain citral, with a yield of 68% calculated by methyl heptenone. In addition, acetylene and methyl heptenone could perform condensation reaction to form dehydrogenation linalool, which was then rearranged in the presence of silicon sulfone catalysis at 140~150 °C in inert solvent to get citral.
Derived from litsea cubeba oil (which is the main method to product citral in China)
Add 30 kg of cubeba oil containing about 75% of citral into a mixture under fully stirring, which was prepared with 18 kg of sodium bicarbonate, 38 kg of sodium sulfite and about 165 kg of water, and then continually stir for 5 to 6 h at room temperature. After standing overnight for stratification, the lower citral precipitated in the form of adduct. And the adduct was then washed with a small amount of toluene to remove oil and dried. And then add 10% sodium hydroxide solution to decompose citral at room temperature, and extract it with benzene. The extract was first distilled at atmospheric pressure (80-82°C) to recover benzene and then distilled under reduced pressure to collect fractions of 110-111°C (1.47kPa) to obtain pure product of 98% citral in an amount of about 15 to 16 kg. |
| Chemical Properties |
mobile light yellow liquid with a lemon-like smell |
| Uses |
Citral is an anti-microbial agent found in plants with antibacterial activity against some food pathogens. It is also a fragrance compound with a distinct lemon scent. |
| Uses |
citral is a naturally occurring aroma compound used to provide a lemon-type fragrance. Citral is a constituent of lemon oil, lemon- grass oil, lime oil, ginger oil, verbena oil, and other plant-derived C essential oils. |
| General Description |
A clear yellow colored liquid with a lemon-like odor. Less dense than water and insoluble in water. Toxic by ingestion. Used to make other chemicals. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
Citral is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Citral can react with alkalis and strong acids. Citral can readily isomerize. |
| Fire Hazard |
Citral is combustible. |
| Contact allergens |
Citral is an aldehyde fragrance and flavoring ingredient, a blend of isomers cis (Neral) and trans (geranial). As a fragrance allergen, citral has to be mentioned by name in cosmetics within the EU. |
| Safety Profile |
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. Experimental reproductive effects. A severe human and experimental skin irritant. Mutation data reported. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. |

 China
China