| HEXAFLUOROETHANE Basic information |
| Product Name: |
HEXAFLUOROETHANE |
| Synonyms: |
1,1,1,2,2,2-Hexafluoroethane;C2F6;ethane,hexafluoro-;Ethylhexafluoride;f116;F-116;FC1160;Fluorocarbon 116 |
| CAS: |
76-16-4 |
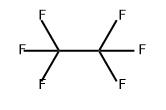
| MF: |
C2F6 |
| MW: |
138.01 |
| EINECS: |
200-939-8 |
| Product Categories: |
refrigerants;Organics |
| Mol File: |
76-16-4.mol |
 |
| |
| HEXAFLUOROETHANE Chemical Properties |
| Melting point |
−100 °C(lit.) |
| Boiling point |
−78 °C(lit.) |
| density |
1,607 g/cm3 |
| vapor density |
4.7 (vs air) |
| vapor pressure |
430 psi ( 21 °C) |
| refractive index |
1.2060 |
| CAS DataBase Reference |
76-16-4(CAS DataBase Reference) |
| Hazard Codes |
Xi |
| Safety Statements |
38 |
| RIDADR |
UN 2193 2.2 |
| WGK Germany |
3 |
| RTECS |
KI4110000 |
| Hazard Note |
Irritant |
| TSCA |
T |
| HazardClass |
2.2 |
| Provider |
Language |
| SigmaAldrich |
English |
| |
| HEXAFLUOROETHANE Usage And Synthesis |
| Chemical Properties |
colourless gas |
| Uses |
Dielectric and coolant, aerosol propellant, refrigerant. |
| General Description |
HEXAFLUOROETHANE is a colorless, odorless gas. HEXAFLUOROETHANE is relatively inert. The mixture is nonflammable and nontoxic, though asphyxiation may occur because of displacement of oxygen. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. |
| Reactivity Profile |
HEXAFLUOROETHANE is chemically inert in many situations, but can react violently with strong reducing agents such as the very active metals and the active metals. Can react with strong oxidizing agents or weaker oxidizing agents under extremes of temperature. |
| Health Hazard |
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases. |
| Fire Hazard |
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket. |
| Purification Methods |
Purify it for pyrolysis studies by passing through a copper vessel containing CoF3 at ca 270o, and hold for 3hours in a bottle with a heated (1300o) platinum wire. It is then fractionally distilled. [Steunenberg & Cady J Am Chem Soc 74 4165 1962, Beilstein 1 IV 132.] |
|

 China
China





