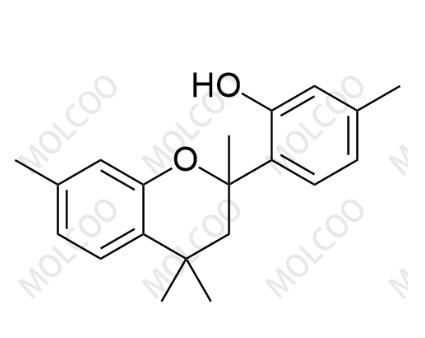
5-methyl-2-(2,4,4,7-tetramethylchroman-2-yl)phenol
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code:B201331
English Name:5-methyl-2-(2,4,4,7-tetramethylchroman-2-yl)phenol
English Alias:Inulavosin; 5-methyl-2-(2,4,4,7-tetramethylchroman-2-yl)phenol
CAS No.:6022-36-2
Molecular Formula:C₂₀H₂₄O₂
Molecular Weight:296.40
High-Purity Reference Standard:Confirmed by HPLC (≥99.0%), NMR (1H, 13C), IR, and elemental analysis, suitable for natural product or phenolic compound impurity analysis and quality control.
Good Stability:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.3% in common organic solvents like methanol and ethanol within 6 months.
Natural Product Research:Serves as a reference for qualitative and quantitative analysis of Inulavosin in Asteraceae plant extracts (e.g., Inula japonica), exploring the material basis of its anti-inflammatory and antioxidant activities.
Drug Impurity Analysis:Used for detection of related impurities in APIs and formulations of phenolic structure-containing drugs, controlling content to meet ICH Q3A standards (single impurity limit ≤0.1%).
Method Validation:Acts as a standard for developing phenolic compound detection methods, verifying UPLC resolution (≥3.0) and LOD (0.01 ng/mL).
5-methyl-2-(2,4,4,7-tetramethylchroman-2-yl)phenol (Inulavosin) is a natural phenolic compound mainly found in Asteraceae plants such as Inula japonica and oregano. The phenolic hydroxyl group and chroman ring in its structure form a conjugate system, potentially endowing it with unique biological activities. Studies have shown that this compound may participate in plant defense mechanisms and attract attention as a potential active ingredient or impurity in drug research and development. As control of phenolic components in natural products is crucial for drug efficacy and safety, its study has gradually become an important part of quality control.
Detection Technology:UPLC-MS/MS with C18 column (1.7μm) and 0.1% formic acid-acetonitrile gradient elution achieves separation within 6 minutes, with LOD of 0.005 ng/mL for trace analysis.
Synthesis and Source:Isolated from Inula japonica ethanol extracts by silica gel column chromatography or synthesized by condensation of phenolic precursors with isoprene derivatives under acidic conditions (e.g., sulfuric acid catalysis). Optimizing reaction temperature (e.g., 50-60℃) and catalyst dosage improves yield.
Biological Activity Research:In vitro experiments show significant anti-inflammatory activity (NO inhibition IC₅₀=18.5 μM) against RAW 264.7 macrophages and better antioxidant capacity (DPPH radical scavenging IC₅₀=15.2 μM) than vitamin E. Related pharmacological mechanism studies are ongoing.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!