1/1
Sulfadiazine
$ 1.00
/1KG
- Min. Order1G
- Purity98%
- Cas No68-35-9
- Supply Ability100KG
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Sulfadiazine |
| CAS No | 68-35-9 |
| EC-No | |
| Min. Order | 1G |
| Purity | 98% |
| Supply Ability | 100KG |
| Release date | 2019/07/06 |
AD68
| Sulfadiazine Basic information |
| description Chemical Properties Pharmacological effects Pharmacokinetics Indications Side effects Uses |
| Product Name: | Sulfadiazine |
| Synonyms: | 4-amino-N-2-pyrimidinyl;4-Amino-N-(2-pyrimidinyl)benzenesulfonamide, N1-(Pyrimidin-2-yl)sulfanilamide;Sulfadiazine zinc salt;Eksadiazine;4-azanyl-N-pyrimidin-2-yl-benzenesulfonamide;Sulfadiazine Base & Sodium;Sulfadiazine,4-Amino-N-(2-pyrimidinyl)benzenesulfonamide, N1-(Pyrimidin-2-yl)sulfanilamide;Sulfadiazine (200 mg) |
| CAS: | 68-35-9 |
| MF: | C10H10N4O2S |
| MW: | 250.28 |
| EINECS: | 200-685-8 |
| Product Categories: | Sulfonamides (Antibiotics for Research and Experimental Use);Intermediates & Fine Chemicals;Isotope Labelled Compounds;Pharmaceuticals;Sulfur & Selenium Compounds;Pharmaceutical intermediate;Sulfadiazine;API;ADIAZINE;Sulfonamides;pharmaceutical;Antibiotics for Research and Experimental Use;Biochemistry |
| Mol File: | 68-35-9.mol |
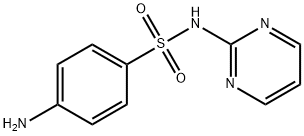 |
|
| Sulfadiazine Chemical Properties |
| Melting point | 253 °C (dec.)(lit.) |
| density | 1.3780 (rough estimate) |
| refractive index | 1.6440 (estimate) |
| storage temp. | 0-6°C |
| solubility | Soluble in dimethyl sulfoxide. |
| color | white |
| Merck | 14,8903 |
| CAS DataBase Reference | 68-35-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulfadiazine(68-35-9) |
| EPA Substance Registry System | Benzenesulfonamide, 4-amino-N-2-pyrimidinyl-(68-35-9) |
| Safety Information |
| Hazard Codes | Xn,Xi |
| Risk Statements | 22-36/37/38-42/43-43-42 |
| Safety Statements | 26-36 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | WP1925000 |
| F | 10 |
| TSCA | Yes |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| Sulfadiazine Usage And Synthesis |
| description | Sulfadiazine is an oral sulfonamide anti-bacterial agent. it is a synthetic pyrimidinyl sulfonamide derivative, The chemical classification of sulfadiazine is Sulfonamides.The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus. Sulfadiazine is used in combination with pyrimethamine to treat toxoplasmosis in patients with acquired immunodeficiency syndrome and in newborns with congenital infections. |
| Chemical Properties | It is White or white-like crystal or powder and is odorless and tasteless. It gradually becomes dark upon exposure to light. It is insoluble in water, soluble in boiling water (1:60), slightly soluble in ethanol and acetone and insoluble in chloroform and ether. It is easily soluble in dilute hydrochloric acid, sodium hydroxide solution or ammonia solution. Its melting point is 252~256 ℃ (decomposition occurs at the same time). Its sodium salt is a white crystalline powder and is odorless with slightly bitter taste. Its sodium salt gradually turns to brown color up exposure to light. When being subject to long-term storage in the moist air, it can slowly absorb carbon dioxide and precipitate out sulfadiazine. It is soluble in water, slightly soluble in ethanol, but insoluble in chloroform and ether. Its 10% aqueous solution has a pH of 9.5 to 10.5. |
| Pharmacological effects | Sulfadiazine hemolytic has inhibitory effect on various kinds of microorganisms including streptococcus, staphylococcus, meningococcus, pneumococcus, Neisseria gonorrhoeae, Escherichia coli, Shigella and other sensitive bacteria as well as Chlamydia trachomatis, actinomycetes, Plasmodium, Toxoplasma gondii and Star Nocardia. The antibacterial activity of this product is similar as that of sulfasuccinamide. But in recent years, there are increased reports regarding the bacterial resistance to this product, especially in Streptococcus, Neisseria and Enterobacteriaceae. Sulfa-class belongs to broad-spectrum antibacterial agents. The molecular structure of sulfadiazine is similar as that of the amino benzoic acid (PABA), and can compete with PABA for acting on the dihydrofolate synthetase inside bacterial cells, thereby preventing the biosynthesis of folic acid (essential for bacteria) using PABA as the raw material and further reducing the amount of metabolically active folate, which is a indispensible material for bacterial synthesis of purines, thymidine and deoxyribonucleic acid (DNA) and thereby inhibiting the growth and reproduction of bacteria. |
| Pharmacokinetics | This product can be easily absorbed after oral administration (more than 70% of the administrated drug can be absorbed), but with the absorption rate being relative slow. After a single oral dose of 2g, the plasma concentration reaches peak after 3~6 hours with the peak of free plasma concentration being about 30~60μg/ml. After drug absorption, it widely distributed in body tissue and pleural fluid, peritoneal fluid, synovial fluid, aqueous humor, saliva, sweat, urine and bile. The drug is easy to penetrate through the blood-brain barrier as well as being able to enter into the breast milk and penetrate through the placental barrier. When there is no meningeal inflammation, cerebrospinal fluid drug concentration is about 50% of the plasma concentration. While the value can be 50% to 80% when there is meningeal inflammation. The drug has a low plasma protein binding rate which is around 38% to 48%. The elimination life for patients of normal renal function is about 10 hours while it can be as long as 34 hours for patients with kidney failure. Sulfadiazine drug is mainly deactivated in the liver through acetylation metabolism, followed by deactivation upon binding to the glucuronide in the liver. The drug is primarily excreted through glomerular filtration. Within 48 to 72 hours after administration of the drug, around 60% to 85% of administrated drug is excreted form urine. In addition, there is still a small amount of drugs being able to be discharged through feces, milk, and bile. Hemodialysis can partially clear the drug. However, peritoneal dialysis can’t effectively remove the drugs. |
| Indications | Sulfa drugs belong to broad-spectrum antibiotic. However, because of the resistance of many current clinical common pathogens to this class of drugs, it is only used for treating the infection caused by sensitive bacteria and other kinds of susceptible pathogens. Sulfadiazine (not including the FDC of this drug together with trimethoprim) has its main indications as follows: 1. Used for prevention and treating the meningococcal meningitis caused by sensitive Meningococcal. 2. When being used in combination with trimethoprim, it can be used for treating the otitis media and other kinds of soft tissue infection caused sensitive Haemophilus influenzae, Streptococcus pneumoniae and other kinds of Streptococcus. 3. Used for treating disease related to star nocardia. 4. Used as the adjuvant drug assisting in the treatment of chloroquine-resistant falciparum malaria. 5. Used as the secondary-choice drug for treatment of Chlamydia trachomatis-induced urethritis and cervicitis. 6. Used as the secondary-choice drug for treatment of neonatal inclusion conjunctivitis caused by Chlamydia trachomatis. |
| Side effects | 1. it can cause kidney damage. This product has a low acetylation ratio. This product and its acetylated compound has a low solubility in the urine and is easily subject to crystal precipitation upon a acidic urine, doing harm to the renal tubular as well as the epithelial cells of other urinary tract and causing crystallization of urine, hematuria, proteinuria, and even urine retention or uremia in severe cases. 2. hematopoietic system reactions include neutropenia, acute hemolytic anemia, aplastic anemia, and thrombocytopenia purpura. 3. gastrointestinal reactions include nausea and vomiting. Occasionally: jaundice, liver and spleen. For newborns, premature children, it can cause jaundice, and even kernicterus. 4. urinary system damage: As the prototype of sulfonamides and acesulfame are primarily subject to renal excretion and thus have higher concentrations in the urine. Upon acidic urine, its solubility decreases and can be crystallized and precipitated in renal tubules, renal pelvis, ureter or bladder, causing crystallization of urine, hematuria, proteinuria, dysuria, oliguria and even urine retention. 5. allergic reactions: commonly include rash, drug fever and even exfoliative dermatitis, erythema multiforme exudativum in severe cases. This often occurs during the 7 to 10 days after the medication. Photosensitive dermatitis has also been reported. |
| Uses | It is excellent kinds of sulfa drugs with strong antibacterial activity, good efficacy, rapid absorption, slow excretion rate and high plasma concentration. It is clinically used for treating upper respiratory tract infection, Meningococcal meningitis, otitis media, boils carbuncle, puerperal fever, urinary tract infections and acute dysentery and so on. It is a sulfa-type drug which is used for the treatment of infection caused by hemolytic streptococcus, pneumococcus, meningococcis, Neisseria gonorrhea, and E. coli. It is a sulfa drug with antibacterial effect and convergence effect. |
| Chemical Properties | White to slightly yellow crystalline pow |
| Uses | Antibacterial. |
| Uses | DMSO soluble potent immunosuppressant, neuroprotective neuroregenerative, in vitro T cell proliferation blocker. disrupts calcineurin-mediated signal transduction in T lymphocytes |
| Definition | ChEBI: A sulfonamide consisting of pyrimidine with a 4-aminobenzenesulfonamido group at the 2-position. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;



