| Antitumor antibiotics |
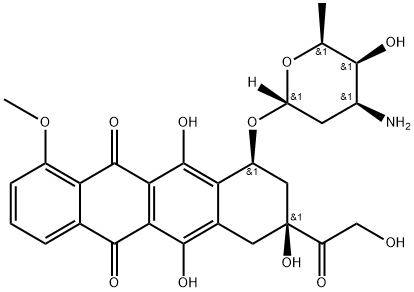
Adriamycin is a kind of anthracycline antitumor antibiotics produced by Streptomyces peucetius sub sp caesius, belonging to cell cycle non-specific drug (CCNSA). Cells of S and M phases are most sensitive to it. Its hydrochloride appears as orange needle crystal. The melting point is 204 ~ 205 ℃. It is easily soluble in water, ethanol and methanol. The aqueous solution is stable and stays constant at 5 ° C for 1 month but is not stable at higher temperatures or in acidic or alkaline solutions. The acidic aqueous solution is orange, neutral orange-red, alkaline (pH> 9) purple-blue. It is insoluble in acetone, benzene, petroleum ether, ether and chloroform. It is an antitumor antibiotic isolated from the actinomycetes culture. The mechanism of action is similar to daunorubicin, which has a broader anti-tumor spectrum and a higher therapeutic index. It is clinically applied for the treatment of acute and chronic lymphocytic leukemia and solid tumor leukemia, lymphoma, breast cancer, ovarian cancer, soft tissue sarcoma, osteogenic sarcoma, rhabdomyosarcoma, Ewing's sarcoma, nephroblastoma, neuroblastoma, gastric cancer, pancreatic cancer, liver cancer, prostate cancer, head and neck squamous cell carcinoma, testicular cancer, lung cancer, bladder cancer, medullary thyroid carcinoma and sarcoma. This product can cause myelosuppression, gastrointestinal reactions and cardiac toxicity. 4'-Epirubicin (4'-epi-adriamycin) is the reverse configuration of the C4 hydroxyl in the glycosyl group of Adriamycin. Its anti-tumor effect is similar to that of Adriamycin, but its cardiotoxicity is low. 4'-deoxyadriamycin (4'-deoxyadriamycin) has a different antitumor spectrum of animal tumors with doxorubicin with lower damage to the heart compared to doxorubicin. |
| Pharmacological effects |
This product belongs to anthracycline antineoplastic drugs, having similar structure to daunorubicin with the only difference being an H-hydroxy substitution in the side chain 14 carbon atoms. It has both fat-soluble anthracene ring ligand and water-soluble daunosamine. It also has acidic phenolic hydroxyl and basic amino group, thus having a strong anticancer pharmacological activity. Adriamycin is obtained from the culture Str. Pe-ucetius var. Caesius. Owing to the quinone-hydroquinone structure on the anthracene ring ligand in the molecule, it has the ability of accepting electrons and providing neutrons, to be inserted between adjacent base pairs of DNA, generating active free radicals, unwinding and breaking DNA double helix. It can further inhibit the activity of the nucleic acid template, interfering with the transcription process to prevent mRNA synthesis. In addition it may cause cell membrane rupture, exhibiting cytotoxicity, belonging to a cell cycle non-specific drug. This product has the function of forming superoxide radicals, and has a special role in the destruction of cell membrane structure and function. It is effective in the targeting all stages of the cells, but being of highest efficient in the treatment of the early S phase cells, followed by M, with the G1 phase being most insensitive to it. It can delay the G1, S and G2 phase. However, the maximum cytotoxicity occurs in S phase and is more sensitive to early S and M phase cells. Adriamycin can cause chromosome aberrations in cells and increase the exchange rate of chromatids.
This product has poor oral absorption, and should be administrated by only intravenous injection. Its plasma protein binding rate is low. It can rapidly disappear from the blood after injection, being widely distributed in the heart, stomach, lung, liver and spleen organizations, but no penetrating through the blood-brain barrier. Plasma attenuation curve is divided into three phases with half-life being 12 minutes, 3.3 hours and 30 hours, respectively. It is mainly subject to liver metabolism with the main metabolite being doxorubicin alcohol, having a considerable anti-cancer activity. Approximately 40% to 50% of the dose is excreted by bile after 7 days of administration, of which 50% is prototype and 23% is active metabolite. 5% to 10% of urine excreted. Upon liver and renal insufficiency, doxorubicin stay in the body for longer with the clear curve exhibiting heterogeneous phases and its three-phase T1 / 2 were 0.5 hours, 3 hours and 40 to 50 hours, respectively. It is clinical mainly for the treatment of acute or chronic leukemia, Hodgkin and non-Hodgkin's lymphoma and malignant lymphoma. Daunorubicin resistant tumors are still sensitive to doxorubicin. It also has certain efficacy in the treatment of Ewing's tumor, osteosarcoma, soft tissue tumor, lung cancer, choriocarcinoma, breast cancer, bladder cancer, thyroid cancer and soft tissue tumors. It combination with cytarabine, vincristine and fluorouracil can increase its efficacy. Tumor cells are resistant to doxorubicin. Drug-resistant cells, compared to the sensitive cells, have lower permeability of the cell membrane, decreased cellular uptake doxorubicin reduction, and can actively discharge doxorubicin. Daunorubicin resistant cells may also be cross-resistant to doxorubicin. |
| Adverse reactions |
A dose of more than 500mg / m2, 25% to 30% can possibly lead to ECG abnormalities, arrhythmia and decreased cardiac function. There are few cases of congestive heart failure, myocardial degeneration, local necrosis, and even death. 60% to 80% may have myelosuppression, manifested as leukopenia, thrombocytopenia and anemia with reaching minimum in 7 to 10 days and recovering at the first 4 weeks. In addition, there may be nausea and vomiting, mouth ulcers. Similar to daunorubicin, side effects of doxorubicin include cardiotoxicity, bone marrow suppression, nausea, vomiting and stomatitis as well as hair loss. Early signs of cardiac toxicity exhibit temporary ECG changes with the majority undergoing self-remission. Late (chronic) cardiotoxicity, also known as delayed cardiomyopathy that is dose related, is irreversible serious myocardial disease with severe cases being lethal. Therefore, heart disease and hypertension patients should use with caution. There are phlebitis, skin pigmentation and liver damage. Cardiotoxicity patients can administrate vitamin E, vitamin C, coenzyme Q10, N-acetyl cysteine and selenium preparations. Heart failure patients may be given digitalis preparations and diuretics. |
| Medicine interactions |
- Cross-resistant to daunorubicin, vincristine and actinomycin D.
- Synergistic effect with cyclophosphamide, fluorouracil, methotrexate, chlorelimide, cisplatin and nitrosoureas.
- Used with caution during live virus vaccination.
- Combination with azathioprine or mercaptopurine can increase doxorubicin liver toxicity.
|
| Uses |
Doxorubicin (adriamycin) is the most extensively studied of a family of highly fluorescent anthracycline antibiotics produced by several Streptomyces species, first reported in 1967 and later approved for human therapeutic use as an antitumour agent for the treatment of a wide range of cancers. Doxorubicin also exhibits anti-HIV and antibacterial activity. The mode of action of doxorubicin is thought to be due to intercalation of DNA and inhibition of nucleic acid synthesis. |

 China
China