1/1
Lamivudine
$ 1.00
/1KG
- Min. Order1G
- Purity98%
- Cas No134678-17-4
- Supply Ability100KG
- Update time2019-07-06

career henan chemical co
VIP8Y
 China
China
Since:2014-12-17
Address:Zhengzhou High tech Zone, Henan Province, China
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Lamivudine |
| CAS No | 134678-17-4 |
| EC-No | |
| Min. Order | 1G |
| Purity | 98% |
| Supply Ability | 100KG |
| Release date | 2019/07/06 |
AD68
| Lamivudine Basic information |
| New antiviral drug Methods of production What kind of patients suitable for use Lamivudine Zidovudine Pharmacokinetics drug withdrawal Adverse reactions and side effects Disabling condition Chemical property Uses |
| Product Name: | Lamivudine |
| Synonyms: | LaMivudine(Epivir);4-AMino-1-[(2R,5S)-2-(hydroxyMethyl)-1,3-oxathiolan-5-yl]-2(1H)-pyriMidinone;3TC (-)-1-[(2R,5S)-2-(Hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine;3TC/Epivir-HBV;4-AMino-1-((2R,5S)-2-(hydroxyMethyl)-1,3-oxathiolan-5-yl)pyriMidin-2(1H)-one;LaMivudine-13C-d2;cis-Lamivudine;(-)-BCH-189 |
| CAS: | 134678-17-4 |
| MF: | C8H11N3O3S |
| MW: | 229.26 |
| EINECS: | 1806241-263-5 |
| Product Categories: | API;DENDRID;Piperazines;Active Pharmaceutical Ingredients;Antivirals for Research and Experimental Use;Biochemistry;Chemical Reagents for Pharmacology Research;Nucleosides and their analogs;Nucleosides, Nucleotides & Related Reagents;Anti-virals;Intermediates & Fine Chemicals;Pharmaceuticals;API's;HIV/AIDS/Related Products;Sulfur & Selenium Compounds |
| Mol File: | 134678-17-4.mol |
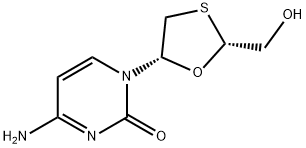 |
|
| Lamivudine Chemical Properties |
| Melting point | 177 °C |
| alpha | D21 -135° (c = 0.38 in methanol) |
| refractive index | -142 ° (C=1, MeOH) |
| Fp | 9℃ |
| storage temp. | Freezer |
| solubility | water: soluble10mg/mL, clear |
| color | white to beige |
| Merck | 14,5352 |
| InChIKey | JTEGQNOMFQHVDC-NKWVEPMBSA-N |
| CAS DataBase Reference | 134678-17-4(CAS DataBase Reference) |
| Safety Information |
| Risk Statements | 63-36/37/38 |
| Safety Statements | 26-36 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| RTECS | UW7361333 |
| Hazardous Substances Data | 134678-17-4(Hazardous Substances Data) |
| Lamivudine Usage And Synthesis |
| New antiviral drug | Lamivudine is a new antiviral drug, belonging to nucleoside reverse transcriptase inhibitors, having a strong inhibitory effect in vitro and animal experimental infection of hepatitis B virus (HBV), can inhibit the synthesis of HIV virus; the drug produced by the GlaxoSmithKline Co. In the early 90s, they are used for the treatment of AIDS drugs in Europe and North American countries. In the middle of the 1990 medical experts found that they have inhibition to hepatitis B virus DNA, in 1998 the United States Food and Drug Administration (FDA) approved the first drug for treatment of hepatitis B treatment. In China, the State Food and Drug Administration approved the drug import mainly used as medicine in the treatment of hepatitis B, Chinese product name as "He Puding". In 1999, officially began on the mainland. Chinese sold after 10 years of clinical verification, lamivudine is the only proven to delay Hepatitis cirrhosis progress, fewer side effects, less cost of medication, currently has 2 million of the country's hepatitis B patients are using. Lamivudine can be metabolized to lamivudine three phosphate in HBV infection cells and normal cells, it is the active form of lamivudine, both inhibitors of HBV polymerase, and is polymerase substrate. Lamivudine three phosphate incorporation into viral DNA chain, can block the synthesis of viral DNA, and does not interfere with normal cell deoxynucleoside metabolism, having weak inhibition to mammalian DNA polymerase alpha and beta, almost no effect on mammalian cell DNA content, and without obvious toxicity to the structure of mitochondria, content and function of DNA. For Serum HBV-DNA detection results of most hepatitis B patients showed that lamivudine can rapidly inhibit the replication of HBV, its inhibitory effect lasted for the entire treatment process. At the same time the serum transaminase decreased to normal, long-term use can significantly improve the liver necrosis and inflammatory changes in relieve or prevent liver fibrosis. |
| Methods of production | Select 6-o-sulfo acylation reaction to the compounds (I), then acetylated to obtaine compound (II), the yield was 96.7%. The compound (II) uses acetic acid as solvent, and 3 moL hydrogen bromide/L acid (45%, W/V) reaction, bromination to get compound (III), the yield was 99%. Bromide (III) and 3.3 moI ethyl potassium xanthate, refluxing in acetone, thio and cyclization; then in methanol with ammonia hydrolysis to obtain the compound (IV), the second step yield is 72%. Compounds (IV) is purified by column chromatography, is crystalline solid. Compound (IV) uses 1.4 mol sodium periodate treatment to make 2,3-CIS glycol open; followed by sodium borohydride reduction to aldehydes and shrinkage the keto form to protect the glycol, and obtain compound (V), the yield is 60%. The compound (V) silane to protect the rest of the primary alcohol, and then take off ketal to obtain compound (VI) and yield is 63%.Use lead tetraacetate to oxidize glycol of compounds (VI), and then to use dichromic acid pyridine salt to further oxidation, and obtain compound (VII), the oxidation method does not affect the sulfur. compounds (VII) is oxidized by using lead acetate to obtain compounds (VIII), the yield is 66%[ according to compounds (VI)]. Compounds (VIII) and (IX) are in the dichloroethane,and TMSOTf as a Lewis acid catalyst, condensated to compound (x), the yield is 64%. And the amount of isomers of compounds (x) is half of the compounds (x) and their available silica gel chromatography to isolate. Compounds (x) in ammonia-methanol and acetyl, the yield is 73% ; with Tetrabutylammonium fluoride solution to remove the silylation to obtain lamivudine and yield of 75%. |
| What kind of patients suitable for use Lamivudine | Not all patients with hepatitis B are suitable for the use of lamivudine. Lamivudine should be applied in the following cases according to the action characteristics of lamivudine and clinical summary of the experience in the application of lamivudine: (1) HBVDNA quantitative detection of moderate positive; HBeAg positive; ALT increased 2~10 times. (2) HBeAg negative, but HBVDNA was moderately positive (do not take the qualitative PCR results as the standard, to do quantitative measurement), ALT increased 2~10 times. The majority of this situation may be the virus gene mutations in the C region. Not suitable for the use of lamivudine is the main: (1) HBVDNA negative or quantitative determination of <105copies/ml; (2)with normal ALT (mainly refers to the virus asymptomatic HBV carriers (ASC); for the past elevated ALT, but now ALT and AST normal patients, can be temporary not treatment, elevated ALT and treated. The patient was not suitable for use, not because lamivudine is harmful to the people, but because of the low effective rate of these patients, does not meet the pharmaceutical economics principle. Lamivudine, like any other drugs, can be used properly to benefit most patients, and inappropriate use will not produce the desired effect, and inappropriate withdrawal may even lead to exacerbations. The above information is Chemicalbook Hanya edited. |
| Zidovudine | Zidovudine (azidothymidine, AZT) is a thymidine analogue. Within the virus-infected cell, the 3′-azido group is used by retroviral reverse transcriptase and incorporated into DNA transcription, preventing viral replication. The shared mechanism of action is inhibition of RNA-dependent DNA polymerase (reverse transcriptase). This enzyme is responsible for conversion of the viral RNA genome into double-stranded DNA before it is integrated into the cell genome. Because these actions occur early in replication, the drugs tend to be effective for acute infections but are relatively ineffective for chronically infected cells. Cellular α-DNA polymerases are inhibited only at concentrations 100-fold greater than those necessary to inhibit reverse transcriptase, thus rendering this drug relatively safe to host cells. Cellular γ-DNA polymerase, however, is inhibited at lower concentrations. AZT is effective against a variety of retroviruses at low concentrations. Resistance to AZT is associated with point mutations resulting in amino acid substitutions in the reverse transcriptase. Prolonged use of AZT can facilitate viral resistance. The risk of resistance also appears to correlate with CD4 cell count and the state of infection. Viral susceptibility to AZT may return after the drug has been discontinued for a period of time. |
| Pharmacokinetics | After oral administration of lamivudine, it is well absorbed. And about 0.1 g of adult oral about 1hr reached peak plasma concentration Cmax 1.1-1.5 u g/ml, bioavailability is 80-85%. and at the same time of food taking, the Tmax delayed 0.25-2.5 HR and lower 10-40% of Cmax, but the bioavailability is unchanged. Intravenous administration research results table Ming lamivudine average distribution capacity is 1.3 L/Kg, system average clearance rate of 0.3 L/h/kg, seventy percent by organic cation transport system and renal clearance and elimination half-life is 5-7hr. within the therapeutic dose range and lamivudine pharmacokinetics showed a linear relationship, the plasma protein binding rate is low. In vitro studies have shown that with serum albumin binding rate is <16-36%. It can pass through the blood brain barrier into the cerebrospinal fluid. Mainly the prototype drug excretion by the kidneys, renal excretion accounted for about total removal of 70% or so, only 5-10% is metabolized into Anti sulfur oxide derivatives. For patients with renal insufficiency can affect lamivudine excretion, and for creatinine clearance rate <30mL/ points in patients, does not recommend the use of this product. Liver damage does not affect the metabolism of drugs, due to increased age and decreased renal excretory function in elderly patients, lamivudine metabolism without significant changes, Only in creatinine clear except rate <30mL/ time-sharing, there is influence. |
| drug withdrawal | Lamivudine is not hepatitis b curative drugs, can only make it better, and easy to rebound after discontinuation of lamivudine treatment. So how long is more appropriate, should according to the therapeutic effect and It differs from man to man. The most accepted standard of withdrawal is: before treatment, HBVDNA positive, HBeAg positive, ALT increased more than 1 times; treatment after HBVDNA seroconversion, HBeAg seroconversion ("big Sanyang" to "small Sanyang"), ALT normalization, maintain the effect of the above 6 months can be stopped. Of course in this standard withdrawal is not only without recurrence, the recurrence rate is low. It is reported that this group of patients discontinued 21 months still maintain curative effect. The recurrence rate of 81%. Asians is slightly higher, but in a year when the recurrence rate is lower than that of 40%. For example not up to this effect and withdrawal, while the majority of cases in a short period of time to recurrence, even some patients because of inappropriate drug withdrawal and lead to illness. So stoping the drug is a great event, I hope the patients in the decision to stop the medicine to go to the hospital to consult an experienced specialist, and not to stop the medicine. |
| Adverse reactions and side effects | Lamivudine is a kind of nucleoside drugs, by inhibiting or blocking synthesis of the hepatitis B virus DNA to restrain replication of hepatitis B virus, thus gradually clear hepatitis B virus, often are used by doctors to treat acute and chronic hepatitis B. Common side effects are easy to produce drug resistance, side effects, withdrawal difficult, fever, upper respiratory tract infection symptoms, headache, abdominal pain, physical discomfort, diarrhea and other symptoms, but the symptoms are generally mild and in a short time the ego recede. At the same time the State Food and Drug Administration released Thirtieth phase drug adverse reaction information bulletin also alert our medical workers and drug producers wary of lamivudine and telbivudine may cause the risk of rhabdomyolysis, myalgia, weakness, elevated creatine kinase, blood creatinine rise higher. Long-term use is easily virus mutates, more performance is after discontinuation of hepatitis B virus DNA positive rotation or is in a cycle of treatment occurs when the hepatitis B virus positive turn, this is the hallmark of virus mutates, indicating that more serious illness, more difficult to treat. Recent studies also suggest, the side effects of lamivudine is also reflected on the damage to the kidney, the majority of patients will have different degrees of renal toxicity, serious and even renal failure. |
| Disabling condition | 1.Chronic asymptomatic hepatitis B virus carriers were normal and no symptoms, whatever virus replication index or not (both "big Sanyang" and "small Sanyang"), are not taking lamivudine. But it is understood that at present this part of patients taking this medicine is a common phenomenon, which wasted drugs and money. At present the treatment of hepatitis B virus is not resolved, medical scientists is also exploring, the existing methods are not mature. The important reason of lamivudine in treatment of hepatitis B virus are invalid, someone after taking HBVDNA may drop to 103 copies/ml, once the withdrawal and we immediately rebound. We found that people taking more than half a year after discontinuation of HBVDNA negative, less than 1 months back, and HBeAg cannot be negative. Therefore, can't use this medicine to treat hepatitis B virus, unless the liver puncture biopsy confirmed chronic liver. The application can only be considered when the inflammatory pathological changes. 2. Acute severe hepatitis or acute liver failure are in critical condition, the ferocious. At this time the patient's main contradiction is not virus replication, some patients even without virus replication index, threatening the patient's life is liver failure, according to "the government ease the emergency treatment of the subject", then the most important is supportive therapy, such as the input of fresh plasma, albumin, artificial liver support therapy. 3. Acute exacerbation of chronic hepatitis B, if the transaminase is greater than the upper limit of the normal value of 10 times, there are obvious jaundice, or serum bilirubin than 85.5 mmol/L, temporarily not to take lamivudine and other antiviral drugs, and liver, jaundice and reducing enzyme treatment is given priority to, When remission can apply a small amount. Although lamivudine on immune function has strongly influenced not the same interferon but in the acute exacerbation of hepatitis, prevent damage on the immune system. No use is the best policy. 4. Chronic hepatitis B in pregnancy or pregnancy after HBV infection, do not use this product, the main reasons of the effect of lamivudine on the fetus are yet to be elucidated. As both at home and abroad, There are application reports and relatively safe, but experts in China has not yet reached a consensus, as a precaution, temporarily application is a wise choice. |
| Chemical property | White solid obtained from methanol-ethyl acetate. [α]D21-132°(C=1.08, methanol). Or from the boiling ethanol crystallization, melting point is 160~162℃. [α]D21-135°(C=0.38, methanol). |
| Uses | 1. Antiviral drugs for hepatitis B. 2. Antiviral drugs for the treatment of liver and gallbladder diseases. 3. Medicine for liver and gallbladder diseases. |
| Chemical Properties | White Crystalline Powder |
| Uses | A reverse transcriptase inhibitor. Antiviral. |
| Definition | ChEBI: A monothioacetal that consists of cytosine having a (2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl moiety attached at position 1. An inhibitor of HIV-1 reverse transcriptase. |
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;


