| Description |
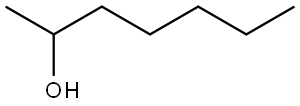
2-heptanol is one isomer of heptanol. It can be used as a flavoring agent and used in perfumery. It is a common agent in analytic chemistry. It can also be used as the intermediate of plasticizer, pharmacy, fragrance and wetting agent and as the co-solvent to be supplemented to nitro-lacquer solvent. |
| References |
[1] Giersch, Wolfgang. "Use of 2,5,6,-trimethyl-2-heptanol as a perfuming and a flavoring agent." (2004).
[2] Weng, Wen Lu, et al. "Vapor–liquid equilibria for nitrogen with 2-hexanol, 2-heptanol, or 2-octanol binary systems." Fluid Phase Equilibria 248.2(2006):168-173.
[3] Whitmore, Frank C., and T. Otterbacher. 2‐Heptanol. Organic Syntheses. John Wiley & Sons, Inc. 2003:60-60. |
| Chemical Properties |
colourless liquid |
| General Description |
A clear colorless alcohol with a mild alcohol odor. Density approximately 6.8 lb / gal and insoluble in water. Hence floats on water. Flash point 130°F. Soluble in most organic liquids. Boiling point 320.7°F. Moderately toxic. Used as a solvent for various resins and as a flotation agent for ore processing. |
| Air & Water Reactions |
Flammable. Insoluble in water. |
| Reactivity Profile |
METHYLAMYL CARBINOL is an alcohol. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. |
| Health Hazard |
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |

 China
China