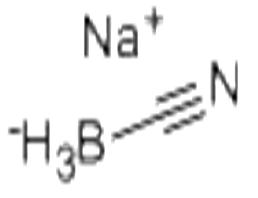
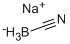| Chemical Properties |
White solid |
| Uses |
- Sodium Cyanoborohydride is a commonly used as a reagent in the reductive amination of aldehydes and ketones and in the reductive alkylation of amines.
- Reagent for selective reductions.
- Used in the synthesis of a novel phenolate-bridged dilanthanum(III) complex of interest as a model for metalloproteins as well as for its importance in basic and applied chemistry.
|
| Uses |
Selective reducing agent for aldehydes, ketones, oximes, enamines; does not reduce amides, ethers, lactones, nitriles, nitro Compounds and epoxides. Also used for reductive amination of ketones and aldehydes, reductive alkylation of amines and hydrazines, reductive displacement of halides and tosylates, deoxygenation of aldehydes and ketones. See Lane, loc. cit. |
| Reducing Agents |
Sodium cyanoborohydride are frequently used for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound.
Contact with strong acids liberates the highly toxic gas HCN. A safer reducing agent with comparable reactivity is sodium triacetoxyborohydride. Reduction with Sodium Cyanoborohydride:
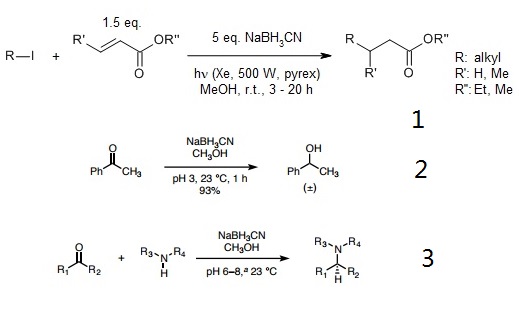
- Tin-free Giese reaction of alkyl iodides with electron-deficient alkenes and the related radical carbonylation process proceeded efficiently in the presence of sodium cyanoborohydride and tetrabutylammonium cyanoborohydride. Transfer of iodine followed by hydride reduction of the resulting carbon-iodine bond is proposed as a possible mechanism.
- Borch and co-workers showed that sodium cyanoborohydride and lithium cyanoborohydride are acid-stable reagents capable of rapidly reducing carbonyl compounds to alcohols at pH 3–4, presumably via a protonated carbonyl cation.
- With care to maintain a pH of 6–7, a mixture of a ketone or aldehyde reactant, an amine, and sodium cyanohydride provides products of reductive amination selectively, without competitive reduction of the carbonyl substrate. Though the conditions of the Borch reduction are mild, sodium cyanoborohydride is highly toxic, as are its byproducts. The pH was maintained by addition of HCl and/or KOH as needed using bromocresol green as an indicator.
 |

 China
China