| Chemical Properties |
WHITE FINE POWDER |
| Uses |
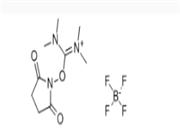
For applications in organozirconium chemistry.1Activates pyrrolidines for improved conversion, via a modified Bouveault reaction, to the corresponding α,α-dimethylamines.2 |
| Uses |
Activates pyrrolidines for improved conversion, via a modified Bouveault reaction, to the corresponding α,α-dimethylamines.1 |
| Definition |
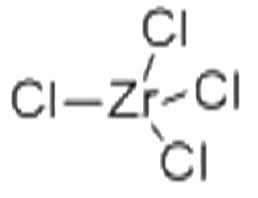
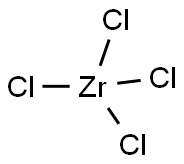
ChEBI: A zirconium coordination entity comprising four chlorine atoms bound to a central zirconium atom. |
| Uses |
Friedel-Crafts catalyst. Component of Ziegler-type catalysts in the condensation of ethylene. Starting material in the synthesis of a number of organic derivatives of zirconium, such as alkoxides and zircocene. The alkoxides have been shown to be of value in the curing of silicone plastic films. The alkoxyzirconium carboxylates are said to be useful in the water-repellent treatment of textiles and other fibrous materials. |
| General Description |
White lustrous crystalline solid. Used as a source of pure zirconium, as a tanning agent, in analytical chemistry and in treating textiles. Zirconium tetrachloride is decomposed by water. Corrosive to metals in the presence of moisture and to tissue. |
| Air & Water Reactions |
Reacts vigorously with water forming hydrochloric acid with the evolution of heat. |
| Reactivity Profile |
Zirconium tetrachloride is corrosive to metals in the presence of moisture and to tissue. Behavior in Fire: Will not burn - sublimes above 626°F (331°C). May give off HCl fumes [USCG, 1999]. Zirconium tetrachloride is incompatible with alcohols (ethanol), lithium metal, and tetrahydrofuran. |
| Health Hazard |
INHALATION: Irritating to upper respiratory tract; presumably caused by liberated HCl. EYES: Irritating. SKIN: Irritating. INGESTION: Burning pain in the mouth and throat, vomiting, watery or bloody diarrhea, retching, collapse, and convulsions. |
| Fire Hazard |
Behavior in Fire: Will not burn - sublimes above 626°F (331°C). May give off HCl fumes. |

 China
China