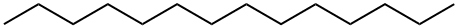| Description |
Tetradecane is an alkane hydrocarbon containing 14 carbon atoms. It is majorly used for the organic synthesis, and used as a kind of organic solvent. Its binary mixtures with hexadecane can be used as phase change materials (PCMs) for cool storage in district cooling systems for refrigeration and air-conditioning. It can also be used as a standard analysis agent in chromatography analysis. In the determination of silicate ion, tetradecane can be used as an internal standard for calibration. |
| References |
He, B. "00/01073 Tetradecane and hexadecane binary mixtures as phase change materials (PCMs) for cool storage in district cooling systems."Fuel & Energy Abstracts 41.2(2000):117–118.
Bassler, Peter, et al. "Olefin is first absorbed from offgas stream formed in oxidation in a solvent comprising hydrocarbons, preferably tetradecane, subsequently desorbed therefrom, possibly freed of aliphatics, and recirculated to oxidation process; recirculation of propene use." US, US 7544818 B2. 2009.
Zhao, M., et al. "Modulated self-assembly of 4,4'- diphenyltetrathiafulvalene molecules on highly oriented pyrolytic graphite by n-tetradecane solvent." Nanotechnology 20.42 (2009):425301.
Chamblee, Theresa S., et al. "Quantitative analysis of the volatile constituents of lemon peel oil. Effects of silica gel chromatography on the composition of its hydrocarbon and oxygenated fractions." Journal of agricultural and food chemistry 39.1 (1991): 162-169. |
| Chemical Properties |
colourless liquid |
| General Description |
Colorless liquid. Must be preheated before ignition can occur. |
| Air & Water Reactions |
Insoluble in water. |
| Reactivity Profile |
Saturated aliphatic hydrocarbons, such as Tetradecane, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. When heated sufficiently or when ignited in the presence of air, oxygen or strong oxidizing agents, they burn exothermically to produce carbon dioxide and water. |
| Health Hazard |
ACUTE/CHRONIC HAZARDS: Explosion hazard: Moderate, in the form of vapor when exposed. |

 China
China