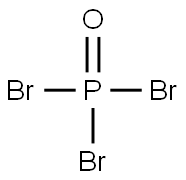| Chemical properties |
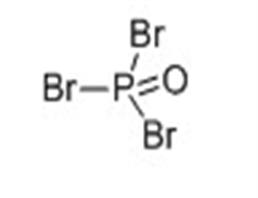
It appears as colorless or pale orange crystal with irritating odor. It has a relative density of 2.822, melting temperature of 56 °C, the boiling point of 191.7 ℃ and the evaporation heat of 38 Kj/mol. It is soluble in ether, benzene, chloroform, carbon disulfide, concentrated sulfuric acid. It can be slowly hydrolyzed to phosphoric acid and HBr in water. |
| Toxicity |
It has similar toxicity to the phosphorus oxychloride. It can react with water, emitting a white smoke-like, irritant and corrosive hydrogen bromide gas which has strong irritation to the eyes, mucous membranes and skin. The patients having inhaled of the vapor or smoke should be sent away from the contaminated area for rest and keeping warm. If eyes are irritated, rinse with water. Upon severe cases, send for medical treatment. Upon skin contact, first rinse with water, and then thoroughly wash with soap. If burned, go for medical treatment. Upon mistakenly administration, immediately gargle and immediately go to the hospital for treatment. |
| Application |
During the chemical process, it can be used as an intermediate and used as the raw materials for manufacturing the bromine flame retardant. |
| Preparation |
Phosphorus oxychloride method: take phosphorus oxychloride as raw material and aluminum trichloride as the catalyst, upon heating at about 80 ℃, introduce hydrogen bromide gas for reaction, generating tribromo phosphorus. The reaction is:
POC13 + 3HBr → POBr3 + 3HCl ↑
The produced hydrogen chloride gas during the reaction, after being absorbed by water, can produce dilute hydrochloric acid.
Phosphorus tribromide method: to a round bottom flask equipped with a reflow condenser, add the mixture of phosphorus pentabromide and phosphorus pentoxide (250 g of PBr5/about 30 g of P2O5) and heat slowly to 150 ° C. Phosphorus pentoxide is a bit more than the phosphorus pentabromide in stoichiometry. At this time, one the one hand, pay attention to expel the bromine out; on the other hand, make sure that the reaction is completed within 5h.
Then, 10 g of bromine and equivalent amount of phosphorus pentoxide were added to the melt and refluxed at 150 ° C for several hours. This is because it takes time for the intermediate product, phosphorus tribromide, to be oxidized to phosphorus pentabromide and transited to tribromonic phosphorus. The resultant was subjected to vacuum distillation under reduced pressure. After removing the initial distillate and the phosphorus tribromide contained therein, the trapping was carried out. The receiver is cooled with ice brine to generate tribromo phosphorus. Its reaction is:
3PBr5 + P2O5 → 5POBr3 |
| Category |
corrosive items |
| Flammability and Hazardous characteristics |
It is subject to decomposition upon coming across water, generating toxic hydrogen bromide gas. |
| Storage characteristics warehouse |
ventilated, low temperature and dry; it should be stored separately from combustible product. |
| Fire extinguishing agent |
Dry powder |
| Chemical Properties |
colorless to grey-brown crystals or fused mass |
| General Description |
A colorless crystalline solid or liquid if heated above 133° F with a pungent odor. Phosphorus oxybromide is commonly heated and shipped in a molten state. Soluble in water, but, decomposed by water to hydrobromic and phosphoric acid with evolution of heat. Reacts with organic materials to cause fire. Evolves highly toxic and corrosive gases when exposed to fire. Corrosive to metals and tissue. Used to make other chemicals. |
| Reactivity Profile |
Phosphorus oxybromide is incompatible with water, strong oxidizing agents, alcohols, bases, including amines. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. |
| Health Hazard |
CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution. |
| Fire Hazard |
EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form. |

 China
China