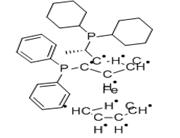
| (S)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]-ETHYLDI-TERT.-BUTYLPHOSPHINE Basic information |
| Reaction |
| Product Name: |
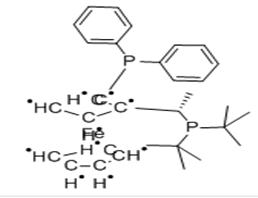
(S)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]-ETHYLDI-TERT.-BUTYLPHOSPHINE |
| Synonyms: |
(S)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]-ETHYLDI-TERT.-BUTYLPHOSPHINE;(S)-(+)-1-[(R)-2-(Diphenylphosphino)ferrocenyl]ethyldi-t-butylphosphine,min.97%;(s,s)-1-[1-(di-tert-butylphosphino)ethyl]-2-(diphenylphosphino]ferrocene (acc to cas);(S)-(+)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]ETHYLDI-T-BUTYLPHOSPHINE, MIN. 97%;(S)-1-[(RP)-2-(Diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine;Josiphos SL-J002-2, (2S)-1-[(1S)-1-[Bis(1,1-dimethylethyl)phosphino]ethyl]-2-(diphenylphosphino)ferrocene (acc to CAS);(2R)-1-[(1S)-1-[Bis(1,1-dimethylethyl)phosphino]ethyl]-2-(diphenylphosphino)ferrocene;(S)-1-[(RP)-2-(Diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine >=97% |
| CAS: |
277306-29-3 |
| MF: |
C27H35P2.C5H5.Fe |
| MW: |
542.461 |
| EINECS: |
|
| Product Categories: |
organophosphine ligand;Chiral Phosphine;Ferrocene Series;Josiphos Series |
| Mol File: |
277306-29-3.mol |
![(S)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]-ETHYLDI-TERT.-BUTYLPHOSPHINE Structure](https://www.chemicalbook.com/CAS/GIF/277306-29-3.gif) |
| |
| (S)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]-ETHYLDI-TERT.-BUTYLPHOSPHINE Chemical Properties |
| alpha |
+412° ±15° (c 0.5, CHCl3) |
| form |
Powder |
| color |
orange |
| Safety Statements |
22-24/25 |
| WGK Germany |
3 |
| F |
10 |
| HS Code |
29319090 |
| |
| (S)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]-ETHYLDI-TERT.-BUTYLPHOSPHINE Usage And Synthesis |
| Reaction |
- Ferrocenylphosphine ligands of the type cpFecp(PR2)(*CH(CH3)PR'2) are a class of asymmetric ligands developed at Solvias in Basel, Switzerland . Ligands of this type are currently used industrially in the stereoselective synthesis of commercial products. A unique feature of these bidentate ligands is the presence of a fixed phosphine moiety and a stereogenic, functionalized side chain, which can be easily modified to accommodate electronic and steric requirements. Based on a versatile synthetic procedure starting with optically active ferrocenes of the type cpFecp(PR2)(*CH(CH3)X) [X = OAc or NR2], a variety of donor atoms can be introduced into the side chain. These ferrocene based phosphine ligands have wide application in the stereoselective hydrogenation of substituted acetamidoacrylates, enol acetates, β-ketoesters and simple alkenes.
- Useful as a ligand in Pd-catalyzed C-N bond-forming reactions.
- Pd-catalyzed enantioselective alkylative desymmetrization of meso-succinic anhydrides.
- Asymmetric hydrogenation of ketones and phosphinylketimines.
- Michael addition of Grignard reagents to ",$-unsaturated esters and thioesters.
- Boration of ",$-unsaturated esters and nitriles.
- Reaction of aryl halides with ammonia.
- Cu-catalyzed reduction of activated C=C bonds with PMHS.
- Regio- and enantioselective hydroboration of vinyl arenes.
- Rh-catalyzed asymmetric ring-opening reactions of oxabicyclic alkenes.
- 1,2-Migrations in Pd-catalyzed Negishi couplings with JosiPhos ligands.
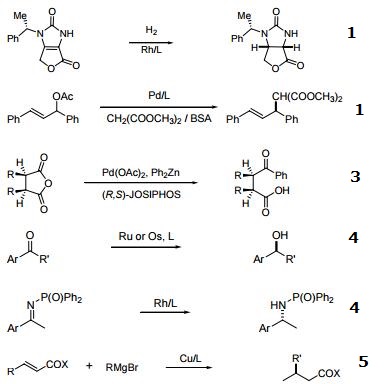
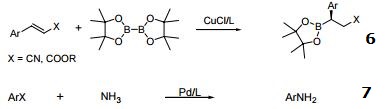
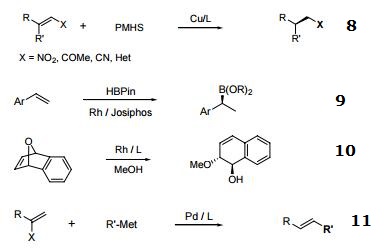 |
| Chemical Properties |
Orange powder |
|

 China
China




![(S)-1-[(R)-2-(DIPHENYLPHOSPHINO)FERROCENYL]-ETHYLDI-TERT.-BUTYLPHOSPHINE Structure](https://www.chemicalbook.com/CAS/GIF/277306-29-3.gif)