| Description |
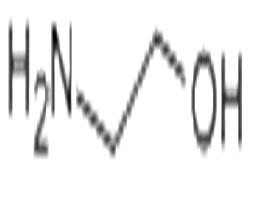
Ethanolamine is a kind of viscous hygroscopic amino alcohol contains both amine and alcohol chemical groups. It is widely distributed inside the body and is a component of lecithin. It has many kinds of industrial applications. For example, it can be used in the production of agricultural chemicals including ammonia as well as the manufacturing of pharmaceuticals and detergents. It can also be used as a surfactant, fluorimetric reagent and removing agent of CO2 and H2S. In pharmaceutical field, ethanolamine is used as a Vascular Sclerosing agent. It also has antihistaminic property, which alleviates the negative symptoms caused by H1-receptor binding. |
| References |
http://www.wisegeek.com/what-is-ethanolamine.htm
https://pubchem.ncbi.nlm.nih.gov/compound/Ethanolamine#section=Top |
| Chemical Properties |
Colorless to yellow liquid |
| Uses |
Used as buffer; removal of carbon dioxide and hydrogen sulfide from gas mixtures. |
| Definition |
ChEBI: A member of the class of ethanolamines that is ethane with an amino substituent at C-1 and a hydroxy substituent at C-2, making it both a primary amine and a primary alcohol. |
| General Description |
A clear colorless liquid with an odor resembling that of ammonia. Flash point 185°F. May attack copper, brass, and rubber. Corrosive to tissue. Moderately toxic. Produces toxic oxides of nitrogen during combustion. |
| Air & Water Reactions |
Water soluble with evolution of heat. |
| Reactivity Profile |
Ethanolamine is a base. Reacts with organic acids (acetic acid, acrylic acid), inorganic acids (hydrochloric acid, hydrofluoric acid, nitric acid, sulfuric acid, chlorosulfonic acid), acetic anhydride, acrolein, acrylonitrile, cellulose, epichlorohydrin, mesityl oxide, beta-propiolactone, vinyl acetate. Emits toxic fumes of nitrogen oxides when heated to decomposition [Sax, 9th ed., 1996, p. 1498]. |
| Health Hazard |
Vapor irritates eyes and nose. Liquid causes local injury to mouth, throat, digestive tract, skin, and eyes. |
| Fire Hazard |
Special Hazards of Combustion Products: Irritating vapors generated when heated. |
| Toxicity |
LD50 orally in rats: 10.20 g/kg (Smyth) |

 China
China