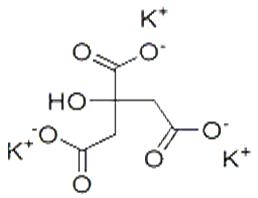
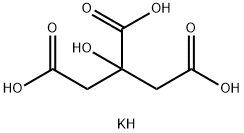
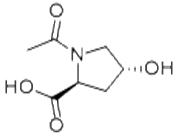
| Potassium citrate |
The applications of potassium citrate (or tripotassium citrate) mainly derive from its alkaline properties. It is used to treat kidney stones composed of uric acid and cysteine, as well as other renal failures caused by excess acidity, including in veterinary applications. As an additive to soft drinks and other edibles, it also functions as an acid buffer, less bitter than other potassium salts, and with less sodium than trisodium citrate.
An additional use of potassium citrate is to supplement potassium in patients with hypokalemia, preventing and treating gout and arrhythmia. |
| Prevention of urinary calculi drug |
Potassium citrate is the drug which is most commonly used to prevent calculus. It was found that in the urine pH value and citric acid value and have a very close relationship with the formation urinary tract calculus. In an acidic urine (pH <5.5), the uric acid solubility is very low, so it is likely to cause uric acid calculus; while in alkaline urine (pH> 7), it is easy to form calcium or magnesium phosphate calculus. 63% of urinary calculus patients are below normal urine citrate, and potassium citrate can provide a lot of citric acid to enhance the urine pH value. So for uric acid calculus, It has a very significant therapeutic effect to low calcium citrate calculus disease and tubular poisoning calcium calculus disease. But It will raise the concentration of potassium and lower the concentration of ammonium ion in urinary, which is because not only complements the citric acid, but also supplements of potassium. Since potassium metabolism is very quickly in the body, there is no change in blood potassium concentration. It has little effect on the body. The daily amount of total urine, oxalate, phosphate, sodium, magnesium, sulfate and uric acid and other aspects test from patients with uric acid calculus, it is found that it is not affected by taking potassium citrate. |
| Chemical properties |
Colorless crystals or white crystalline powder, odorless, cool and salty, slight deliquescence, soluble in water and glycerin, insoluble in ethanol. Tests show that the product had no significant harm to the human body, ADI doesn’t need special provision (FAO/WHO, 1994). |
| Uses |
As a preservative, stabilizing and pH buffering agents and others. China provides for various types of food, according to production needs to use appropriately.
Used in the food, pharmaceutical and other industries, as buffering agents, chelating agents, flavoring agents, stabilizers, antioxidants, emulsifying salts. |
| Production methods |
Citric acid and potassium hydroxide or potassium bicarbonate derived products.
C6H8O7 + 3KHCO3 → CaH5K3O7 · H2O + 3CO2 ↑ + 2H2O |
| Reference |
https://en.wikipedia.org/wiki/Potassium_citrate
https://kidneystones.uchicago.edu/how-citrate-gets-into-the-urine/
https://www.drugbank.ca/drugs/DB09125
http://www.urocit-k.com/
http://www.jungbunzlauer.com/en/products/special-salts/tripotassium-citrate.html
https://www.quora.com/Why-is-potassium-citrate-buffer-is-an-acid-buffer |
| Uses |
For use as an electrolyte replenisher and in the treatment of hypokalemia. |
| Definition |
ChEBI: The anhydrous form of the tripotassium salt of citric acid. |

 China
China