jessie606
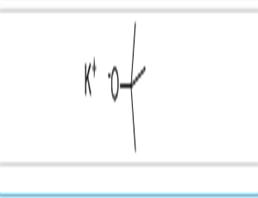
Potassium tert-butoxide
Melting point 256-258 ° C (dec.) (lit.)
Boiling point 275 ° C
Density 0.910 g/mL at 20 °C
Vapor pressure 1 mm Hg (220 °C)
Flash point 54 °F
Storage conditions Flammables area
Solubility Soluble in hexane, toluene, diethyl ether and terahydrofuran.
Form Solution
Color White to off-white
PH value 13 (5g/l, H2O, 20°C)Hydrolysis
Water solubility REACTS
Sensitivity Moisture Sensitive
BRN 3556712
Stable, but reacts violently with water and acids, possibly leading to fire. Incompatible with water, acids, halogenated hydrocarbons, alcohols, strong oxidizing agents, ketones, carbon dioxide.
InChIKey LPNYRYFBWFDTMA-UHFFFAOYSA-N
CAS Database 865-47-4 (CAS DataBase Reference)
EPA Chemical Information 2-Propanol, 2-methyl-, potassium salt (865-47-4)
Potassium tert-butoxide Use and synthesis
Organic Strong Base Potassium tert-butoxide is an important organic base with greater alkalinity than potassium hydroxide. Due to the inductive effect of (CH3)3CO-three methyl groups, it is more alkaline and active than other potassium alkoxides and is therefore a good catalyst. Further, as a strong base, potassium t-butoxide is widely used in organic synthesis such as chemical, pharmaceutical, and agricultural chemicals, such as transesterification, condensation, rearrangement, polymerization, ring opening, and production of heavy metal orthoester. It can be used to catalyze Michael addition reaction, Pinacol rearrangement reaction and Ramberg-Backlund rearrangement reaction; potassium tert-butoxide is used as a condensing agent to catalyze Darzens condensation reaction and Stobbe condensation reaction; it is also a traditional dihalogenated carbenes. The most effective base for the alkoxide-haloform reaction. Therefore, potassium t-butoxide is increasingly favored by the chemical, pharmaceutical, and pesticide industries. The use of potassium tert-butoxide is so extensive that the demand for high-purity potassium t-butoxide is large at home and abroad. However, because its production cost is higher than other alkali metal alkoxides, and the production technology needs to be improved, it is especially important for the in-depth study of potassium t-butoxide.
Related chemical reaction 1, using N-formyl o-toluidine as raw material, reacting with potassium t-butoxide at 350-360 ° C to form hydrazine, separating and then reacting with chloroacetic acid to obtain β-吲哚Acetic acid.
2, β-ring citral is first reacted with propenylmagnesium bromide to form an alcohol, and the obtained alcohol is reoxidized to a ketone, and then isomerized by potassium t-butoxide as a catalyst to become β-purine.
3. The citral and allyl Grignard reagents are used as raw materials to carry out the reaction to form 6,10-dimethyl-4-hydroxy-1,5,9-undecanetriene, which is oxidized under the action of Collins reagent. Production of 6,10-dimethyl-1,5,9-undecanetrien-4-one, isomerization under the action of potassium t-butoxide, biochemical pseudo-damascone, in tetrachlorination Damarone can be obtained by cyclization under the action of tin or phosphoric acid.
4, reacting with 2,6,6-trimethylcyclohex-2-enylformaldehyde and allyl Grignard reagent to form cyclohexenylbutenol, and then oxidizing under the action of copper-zinc catalyst to form a cyclohexane The alkenone can be obtained by isomerization of an alkenylbutenone in an alkali solution of potassium t-butoxide.
5, Darzen reaction: aldehyde, ketone in the presence of an alkaline reagent, with the α-halo acid ester type condensation reaction, while losing hydrogen halide, the formation of α,β-epoxylate. Commonly used The alkaline agents are: sodium ethoxide, sodium amide, sodium metal, potassium t-butoxide, and the like.
6, Stobbe reaction: aldehyde, ketone and succinate in the presence of a basic catalyst (such as sodium ethoxide, potassium t-butoxide, sodium chloride, etc.) similar to aldol condensation reaction, the formation of hydrocarbyl substitution Methylene succinic acid or its monoester.
Strong alkaline condensing agent Potassium tert-butoxide is a strong alkaline condensing agent. Its basicity is stronger than sodium methoxide and sodium ethoxide. It is white or off-white solid powder at normal temperature. Chemical formula (CH3)3C-O-K , molecular weight 112.21, density 0.929, melting point 256-258 ° C, boiling point 275 ° C. Easy to absorb moisture. It is soluble in tert-butyl alcohol, and its solution is relatively stable, and is often used for dehydrohalogenation of halogenated hydrocarbons. Decomposes when exposed to air. Decomposes with water to form potassium oxide and tert-butanol. It can be obtained by reacting t-butanol with potassium metal and then distilling off t-butanol under reduced pressure. Widely used in the condensation, rearrangement and ring opening reactions of organic synthesis, generally using a solution of t-butanol. Potassium tert-butoxide is an organic alkaline corrosive product. It is strongly hygroscopic and should be sealed and stored. Pay attention to fire prevention, sun protection and storage in a cool, ventilated and dry place during storage and transportation.
Method for preparing potassium tert-butoxide in the laboratory. Potassium metal is added to freshly distilled tert-butanol under nitrogen atmosphere, refluxed until the potassium is completely melted, and then kept for 1 hour, and excess t-butanol is distilled off, and the remaining white solid is in the oil bath 180~ Drying under vacuum at 190 ° C for more than 10 h, can obtain crystal powder of potassium t-butoxide, which needs to be stored under nitrogen. It can not be exposed to air & moisture, otherwise it will turn pink. If the yield is calculated by potassium, it is over 99%. The chemical reaction equation for the preparation of potassium tert-butoxide by the reaction of tert-butanol with potassium metal is as follows:
Preparation of chemical reaction equation of potassium tert-butoxide by reaction of tert-butanol with potassium metal
Precautions Potassium tert-butoxide is available in both liquid and solid forms. The liquid industrial product is usually a solution of potassium tert-butoxide in t-butanol. The color of the product is light yellow or milky white, slightly turbid, wherein the potassium and potassium tert-butoxide content is 10% to 12%; the solid product is generally white or off-white powder. The potassium tert-butoxide content is 95% to 97%.
Potassium tert-butoxide is an organic alkaline corrosion product. It is strongly hygroscopic and should be stored in a sealed, cool, dry and ventilated warehouse. Keep away from heat sources when standing, and isolate the fire to prevent exposure to sunlight. Potassium tert-butoxide is highly corrosive to the skin. During loading and unloading, operators should wear protective masks to protect them from corrosion by potassium t-butoxide.
The above information is compiled by Chemicalbook彤彤.
Uses for pesticides, pharmaceuticals, printing and dyeing, catalysts, etc.
Use Potassium tert-butoxide is widely used as a strong base in the condensation, rearrangement and ring opening reactions in organic synthesis such as chemical, pharmaceutical and pesticide.

 China
China