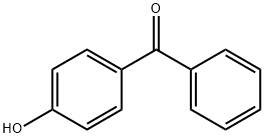
| CAS: | 1137-42-4 |
| MF: | C13H10O2 |
| MW: | 198.22 |
| EINECS: | 214-507-1 |
| Product Categories: | Aromatics, Metabolites & Impurities;Organic Photoinitiators;FINE Chemical & INTERMEDIATES;Aromatic Benzophenones & Derivatives (substituted);Benzophenone;Polymerization Initiators;Aromatics;Metabolites & Impurities;bc0001;1137-42-4 |
| Mol File: | 1137-42-4.mol |
 |
|
| 4-Hydroxybenzophenone Chemical Properties |
| Melting point | 132-135 °C(lit.) |
| Boiling point | 260-262°C 42mm |
| density | 1.1184 (rough estimate) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.5954 (estimate) |
| Fp | 260-262°C/24mm |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 8.14±0.13(Predicted) |
| form | Powder |
| color | White to beige to brown |
| Water Solubility | Insoluble in water. |
| BRN | 777776 |
| LogP | 1.6 at 35℃ |
| CAS DataBase Reference | 1137-42-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Methanone, (4-hydroxyphenyl)phenyl-(1137-42-4) |
| EPA Substance Registry System | Methanone, (4-hydroxyphenyl)phenyl- (1137-42-4) |
| 4-Hydroxybenzophenone Usage And Synthesis |
| Chemical Properties | white to beige fine crystalline powder |
| Uses | Metabolite of benzophenone. |
| Uses | 4-Hydroxybenzophenone is used in the field of organic synthesis. It is used as pharmaceutical intermediate of clomifene citrate. |
| Definition | ChEBI: 4-Hydroxybenzophenone is a member of benzophenones. |
| Preparation | Preparation by condensation of benzotrichloride,
? with phenol in the presence of aluminium chloride in carbon disulfide at 0° (90%) or in the presence of zinc oxide in a water bath;
? with p-bromophenol in 30% sodium hydroxide solution at 80–85° (81%). There is substitution of the bromine atom by the benzoyl group. |
| Synthesis Reference(s) | Journal of the American Chemical Society, 77, p. 4674, 1955 DOI: 10.1021/ja01622a074
The Journal of Organic Chemistry, 19, p. 978, 1954 DOI: 10.1021/jo01371a016
Tetrahedron Letters, 31, p. 3943, 1990 DOI: 10.1016/S0040-4039(00)97513-0 |
| Flammability and Explosibility | Not classified |
| Purification Methods | Dissolve p-benzoylphenol in hot EtOH (charcoal), filter and cool. Alternatively crystallise it once from EtOH/H2O and twice from *benzene [Grunwald J Am Chem Soc 73 4934 1951, Dryland & Sheppard J Chem Soc Perkin Trans 1 125 1986]. [Beilstein 8 IV 1263.] |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
