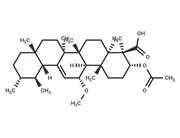
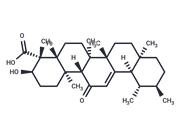
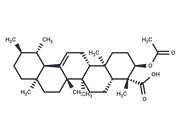
| Name | 3-O-Acetyl-11-hydroxy-beta-boswellic acid |
| Description | 3-O-Acetyl-11-hydroxy-beta-boswellic acid, the precursor of 3-O-acetyl-9,11-dehydro-beta-boswellic acid (a 5-lipoxygenase inhibitor), |
| In vitro | 4 pentacyclic triterpenoid acids were isolated from Boswellia carterii and identified by NMR and Mass spectroscopic analysis (compounds 1, 3-O-acetyl-9,11-dehydro-β-boswellic acid; 2, 3-O-Acetyl-11-hydroxy-beta-boswellic acid ; 3. 3-O- acetyl-11-keto-β-boswellic acid and 4, 11-keto-β-boswellic acid. Their inhibitory activity on Jack bean urease were evaluated. Docking and pharmacophore analysis using AutoDock 4.2 and Ligandscout 3.03 programs were also performed to explain possible mechanism of interaction between isolated compounds and urease enzyme. It was found that compound 1 has the strongest inhibitory activity against Jack bean urease (IC50 = 6.27 ± 0.03 μM), compared with thiourea as a standard inhibitor (IC50 = 21.1 ± 0.3 μM)[1] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline : 2 mg/mL (3.89 mM), Sonication is recommended.
DMSO : 50 mg/mL (97.14 mM), Sonication is recommended.
|
| Keywords | LOX | Lipoxygenase | Inhibitor | inhibit | 5-Lipoxygenase | 3-O-Acetyl-11-hydroxy-β-boswellic acid | 3-O-Acetyl-11-hydroxy-beta-boswellic acid | 3OAcetyl11hydroxybetaboswellic acid | 3-O-Acetyl-11-hydroxy-b-boswellic acid | 3 O Acetyl 11 hydroxy beta boswellic acid |
| Inhibitors Related | 2,5-Di-tert-butylhydroquinone | Nordihydroguaiaretic acid | Caffeic Acid | 5-LOX inhibitor | Diethylcarbamazine citrate | Phenidone | Abietic Acid | Resveratrol | NNK | PF-4191834 | Malotilate | 5-Aminosalicylic Acid |
| Related Compound Libraries | Terpene Natural Product Library | Bioactive Compound Library | Traditional Chinese Medicine Monomer Library | Rare Natural Product Library | Selected Plant-Sourced Compound Library | Anti-Obesity Compound Library | Natural Product Library | Inhibitor Library | Natural Product Library for HTS | Bioactive Compounds Library Max | Traditional Mongolian Medicine Compound Library | Ancient Chinese Classical Formulas Compound Library |

 United States
United States