| CAS: | 1504-06-9 |
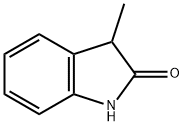
| MF: | C9H9NO |
| MW: | 147.17 |
| EINECS: | 1533716-785-6 |
| Product Categories: | Aromatics, Indole Derivatives, Metabolites & Impurities;Aromatics;Indole Derivatives;Metabolites & Impurities;Building Blocks;C7 to C9;Heterocyclic Compounds;Chemical Synthesis;Heterocyclic Building Blocks;Indoles |
| Mol File: | 1504-06-9.mol |
 |
|
| 3-METHYLOXINDOLE 96 Chemical Properties |
| Melting point | 117-121 °C(lit.) |
| Boiling point | 279.3±29.0 °C(Predicted) |
| density | 1.123±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| pka | 14.81±0.40(Predicted) |
| form | Solid |
| color | Light Beige to Beige |
| Water Solubility | Insoluble in water. |
| CAS DataBase Reference | 1504-06-9 |
| 3-METHYLOXINDOLE 96 Usage And Synthesis |
| Uses | • ;Reactant for enantioselective α-amination reactions1• ;Reactant for aldol reaction with glyoxal derivatives2• ;Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes3• ;Reactant for O-acetylation reactions4• ;Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions5 |
| Uses | A versatile reactant. As 3-Methylindole metabolite. Reactant for enantioselective α-amination reactions, Reactant for aldol reaction with glyoxal derivatives, Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes, Reactant for O-acetylation reactions, Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions. |
| Definition | ChEBI: 3-methyloxindole is a member of the class of oxindoles that is oxindole (1,3-dihydro-2H-indol-2-one) in which one of the hydrogens at position 3 has been replaced by a methyl group. It is a member of oxindoles and a methylindole. |
| General Description | 3-Methyl-2-oxindole (MOI) is a 3-substituted-2-oxindole. It is reported to be formed during the oxidation of indole-3-acetic acid in the presence of FeII under aerobic conditions. MOI undergoes asymmetric anti-Mannich-type reaction with N-tosyl aryl aldimines in the presence of alkaloid cinchona derivatives to form anti-3,3-disubsituted 2-oxindole derivatives. It also undergoes asymmetric hydroxyamination with nitrosoarenes to form N-nitroso aldol products. |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
