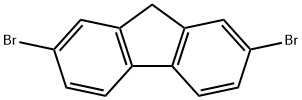
| Description | 2,7-Dibromofluorene is a halogenated polycyclic aromatic compound and its vapour pressure has been measured using the Knudsen effusion method. |
| Chemical Properties | white to off-white crystalline powder |
| Uses | 2,7-Dibromofluorene was used as a template for the N-carbazole capped oligofluorenes which show potential as hole-transporting materials for organic light emitting devices (OLEDs). It may be used in the synthesis of conjugated polymer, poly[9,9′-bis(6′′-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), used in label-free DNA microarrays. It was used in the preparartion of blue photoluminescent unsymmetrically substituted polyfluorene. It was also used in the preparation of 2,7-dibromofluorene monomers containing benzyl ether dendrons (generations 1, 2 and 3) in the 9,9′-position of the fluorene ring, such as:
9,9-bis[(3,5-bis(benzyloxy)benzyloxy)methyl]-2,7-dibromofluorene
9,9-bis[(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy)-methyl] 2,7-dibromo-fluorene
9,9-bis[(3,5-bis(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy) benzyloxy)-methyl]-2,7-dibromofluorene |
| Uses | 2,7-Dibromofluorene is used as a template for the N-carbazole capped oligofluorenes which show potential as hole-transporting materials for organic light emitting devices (OLEDs). It is also used in the synthesis of conjugated polymer, poly[9,9?-bis(6??-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), used in label-free DNA microarrays, in the preparation of blue photoluminescent unsymmetrically substituted polyfluorene and in the preparation of 2,7-dibromofluorene monomers containing benzyl ether dendrons (generations 1, 2 and 3) in the 9,9?-position of the fluorene ring, such as: 9,9-bis[(3,5-bis(benzyloxy)benzyloxy)methyl]-2,7-dibromofluorene, 9,9-bis[(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy)-methyl] 2,7-dibromo-fluorene, 9,9-bis[(3,5-bis(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy) benzyloxy)-methyl]-2,7-dibromofluorene. |
| Uses | 2,7-Dibromofluorene was used as a template for the N-carbazole capped oligofluorenes which show potential as hole-transporting materials for organic light emitting devices (OLEDs). It may be used in the synthesis of conjugated polymer, poly[9,9′-bis(6′′-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), used in label-free DNA microarrays. It was used in the preparartion of blue photoluminescent unsymmetrically substituted polyfluorene. It was also used in the preparation of 2,7-dibromofluorene monomers containing benzyl ether dendrons (generations 1, 2 and 3) in the 9,9′-position of the fluorene ring, such as:
9,9-bis[(3,5-bis(benzyloxy)benzyloxy)methyl]-2,7-dibromofluorene 9,9-bis[(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy)-methyl] 2,7-dibromo-fluorene 9,9-bis[(3,5-bis(3,5-bis(3,5-bis(benzyloxy)benzyloxy)benzyloxy) benzyloxy)-methyl]-2,7-dibromofluorene
|

 Japan
Japan







 Company information
Company information Advantage
Advantage


