| CAS: | 610-99-1 |
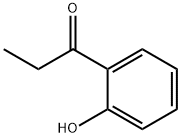
| MF: | C9H10O2 |
| MW: | 150.17 |
| EINECS: | 210-244-1 |
| Product Categories: | Building Blocks;C9;Carbonyl Compounds;Chemical Synthesis;Ketones;Organic Building Blocks;Aromatic Propiophenones (substituted) |
| Mol File: | 610-99-1.mol |
 |
|
| 2'-Hydroxypropiophenone Chemical Properties |
| Melting point | 20-22 °C |
| Boiling point | 115 °C15 mm Hg(lit.) |
| density | 1.094 g/mL at 25 °C(lit.) |
| refractive index | n20/D 1.548(lit.) |
| Fp | >230 °F |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 8.33±0.35(Predicted) |
| form | Oil |
| color | Colourless |
| BRN | 1860264 |
| LogP | 2.540 |
| CAS DataBase Reference | 610-99-1(CAS DataBase Reference) |
| NIST Chemistry Reference | ortho-Hydroxypropiophenone(610-99-1) |
| 2'-Hydroxypropiophenone Usage And Synthesis |
| Chemical Properties | CLEAR BROWNISH LIQUID |
| Preparation | Obtained by Fries rearrangement of phenyl propionate
with aluminium chloride in refluxing carbon disulfide ?, then at 130–150° for 2–3 h after solvent elimination (32–35%)
with aluminium chloride in chlorobenzene using microwave irradiation for 3 ? min at 106° (28%)
with aluminium chloride in nitrobenzene at 50° for 18 h (16%) or at ? 20° for 48 h (10%)
with aluminium chloride in nitromethane at 20° for 7 days (20%)
with aluminium chloride in heptane at 80–90° for 7 h (53%) or in tetrachlo-? roethane at 95° for 5 h (43%)
with aluminium chloride without solvent at 250° for 5 min or at 180–200° for ? 15 min (76–78%), at 140–160° (32–35%), at 140°
with zirconium chloride in o-dichlorobenzene at 120° for 3 h (83%)
with titanium tetrachloride in o-dichlorobenzene at 150° for 1 h (62%) ?, in nitromethane at 20° for 7 days (13%) or without solvent at 50° for 10 h (30%)
with titanium tetrabromide in o-dichlorobenzene at 150° for 1 h (60%)
with stannic chloride at 50° for 3 h (25%)
with boron trifluoride at 120–135° for 15 min (15%)
with zinc chloride at 180–200° for 15 min (10%)
with polyphosphoric acid at 100° (13%)
Also obtained by Friedel–Crafts acylation of phenol with propionyl chloride in the presence of aluminium chloride (40%), at 120–130° for 1 h (45%) according to the method
Also obtained by acylation of phenol with propionic acid
in the presence of boron trifluoride at 165° for 1 h (45%) or at 80° for ? 2 h (8%)
in the presence of polyphosphoric acid at 100° for 10 min (by-product)
in the presence of zinc chloride at 160° for 1 h
Also obtained by isomerization of 4-hydroxypropiophenone with aluminium chloride (1.5 equiv) at 165° for 1 h (40%) or at 180–200° for 15 min (55%)
Also obtained by demethylation of 2-propionylanisole with fuming hydrochloric acid (d = 1.19) in a sealed tube at 110° for 6 h
Also obtained (by-product) by heating 3-tert-butyl-4-hydroxypropiophenone with aluminium chloride at 170° for 15 min (13%)
Also obtained by treatment of 2,3-dimethylchromone with sodium ethoxide in boiling ethanol for 30 h according to the method
Also obtained from 2-allylphenol by treatment with perbenzoic acid in ethyl ether, first at 0°, then between 0° and 25° for 24 h (78%)
Also obtained by reaction of 2-bromophenyl propionate in a ethyl ether/hexane/THF mixture at low temperature (?78 to ?95°) with sec-butyllithium to give, after hydrolysis, the titled ketone (metal-promoted Fries rearrangement) (17%)
Also obtained by reaction of ethylmagnesium bromide
with 2-hydroxybenzamide in boiling benzene, followed by hydrolysis ? (30%)
with 2-hydroxy-N,N-diethylbenzamide in boiling benzene, followed by ? hydrolysis (82–84%)
Also isolated by heating of 1-(o-hydroxyphenyl)cyclopropyltrimethylam-monium iodide for 1 h at 140° with 1.5 equiv of N,N-diisopropylethylamine (not water-free) (38%)
Also obtained by hydrolysis of 2-(1-methyliminopropyl)phenol (4aa) with aqueous acetic acid/THF at 40° for 4 h (86%)
Also obtained by photolysis of phenyl propionate in water or in solution containing b-cyclodextrine (254 nm) at 25° for 2 h
Also obtained by treatment of 2-(1-hydroxypropyl)phenol with MnO2 in methylene chloride for 7 h at r.t. |
| Definition | ChEBI: 2'-hydroxypropiophenone is an aromatic ketone. |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock










 China
China

 Company information
Company information Advantage
Advantage


