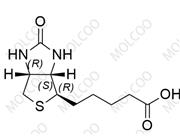
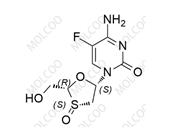
2-epi Emtricitabine 145416-34-8
2-Epi-Emtricitabine Reference Standard
2-Epi-Emtricitabine is a crucial pharmaceutical impurity and an isomer of Emtricitabine. As an antiretroviral drug, Emtricitabine and its related impurities play a vital role in drug research and quality control.
Our 2-Epi-Emtricitabine reference standards are meticulously prepared and rigorously tested to ensure their high purity and chemical stability. These reference standards are essential tools in drug research and development, drug metabolism studies, impurity profiling, and drug quality control.
By using our 2-Epi-Emtricitabine reference standards, you can easily achieve the following goals:
Precise Analysis: Accurately determine the content of 2-Epi-Emtricitabine in drugs to ensure their quality.
Impurity Control: Effectively monitor impurity levels in drugs to safeguard their safety and effectiveness.
R&D Support: Provide powerful impurity analysis support for new drug research and development, accelerating the drug discovery process.
Our 2-Epi-Emtricitabine reference standards are compatible with various drug analysis platforms, including High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), and Mass Spectrometry (MS), ensuring accurate and reliable analysis results across different application scenarios.


 China
China