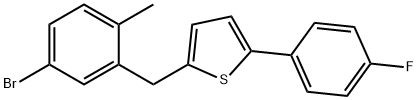
| Synthesis | prepares 2-(4-fluorophenyl) thiophene III:
Prepared by Grignard reagent: N 2under protection; magnesium chips (11.7g is added in the 500mL there-necked flask that thermometer, reflux condensing tube, constant pressure funnel be housed; 480mmol), THF (15.0mL), drips 2 p-Fluoro bromo benzenes; add the initiation reaction of 2 iodine post-heating; p-Fluoro bromo benzene (70.0g, 400mmol) is dissolved in THF (250mL), after under reflux, drip the THF solution of p-Fluoro bromo benzene; drip Bi Baowen backflow 2h, GC detection feedstock conversion complete.
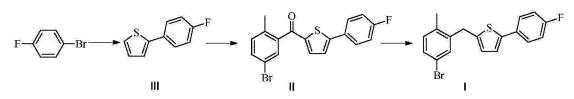
Linked reaction: N 2under protection; in the 1000mL there-necked flask that thermometer, reflux condensing tube, constant pressure funnel be housed, add 2-bromothiophene (52.2g, 320mmol), under stirring, add two (methyl ethyl diketone) palladium (23.4mg; 0.0320mmol); THF (200mL), is heated to 50 DEG C, the above-mentioned Grignard reagent prepared of rear dropping; drip to finish and be warming up to 60 DEG C; insulation 1h, GC detect to raw material reaction complete, terminate reaction.System is cooled to 30 DEG C, drips dilute hydrochloric acid (2M, 150mL) under ice-water bath, drips complete vigorous stirring 0.5h, removes ice-water bath and make system naturally rise to room temperature, stratification, water layer CH 2cl 2(100mL × 3) extract, merge organic phase saturated sodium-chloride 200mL to wash, rear drying, concentrating under reduced pressure obtain light tan solid, vacuum-drying to constant weight obtains 2-(4-fluorophenyl) thiophene III (57.4g, 312mmol), it is 98.5% that GC detects purity, and yield is 99.1%.
1H NMR(500MHz,CDCl 3)δ7.65~7.54(m,2H),7.33~7.22(m,2H),7.14~7.03(m,3H).Gc-Ms:178.1. |

 Japan
Japan







 Company information
Company information Advantage
Advantage