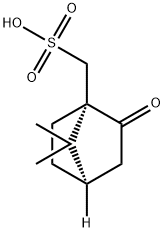
| CAS: | 3144-16-9 |
| MF: | C10H16O4S |
| MW: | 232.3 |
| EINECS: | 221-554-1 |
| Product Categories: | chiral;Pharmaceutical Intermediates;FINE Chemical & INTERMEDIATES;Chiral Compounds;Bicyclic Monoterpenes;Biochemistry;for Resolution of Bases;Optical Resolution;Reagents for Oligosaccharide Synthesis;Synthetic Organic Chemistry;Terpenes;Peptide;Sulfur & Selenium Compounds;3144-16-9;3597-91-9 |
| Mol File: | 3144-16-9.mol |
 |
|
| (1S)-(+)-Camphor-10-sulphonic acid Chemical Properties |
| Melting point | 196-200 °C (dec.)(lit.) |
| alpha | 22 º (589nm, c=20, H2O 25 ºC) |
| Boiling point | 344.46°C (rough estimate) |
| density | 1.2981 (rough estimate) |
| refractive index | 21.5 ° (C=5, H2O) |
| storage temp. | Store below +30°C. |
| solubility | Exhibit moderate solubility in HCCl. |
| pka | 1.17±0.50(Predicted) |
| form | Crystalline Powder |
| color | White to off-white |
| PH | 0.3 (200g/l, H2O) |
| optical activity | [α]20/D +21.5±1°, c = 10% in H2O |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,1734 |
| BRN | 2809675 |
| Stability: | Stable. Protect from moisture. Incompatible with strong oxidizing agents, strong bases. |
| InChIKey | MIOPJNTWMNEORI-GMSGAONNSA-N |
| CAS DataBase Reference | 3144-16-9(CAS DataBase Reference) |
| EPA Substance Registry System | Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 7,7-dimethyl-2-oxo-, (1S,4R)- (3144-16-9) |
| (1S)-(+)-Camphor-10-sulphonic acid Usage And Synthesis |
| Synthesis | (1S)-(+)-Camphor-10-sulphonic acid can be obtained from natural camphor by brominated sulfonation . |
| Chemical Properties | white to light beige powder |
| Uses | (1S)-(+)-Camphor-10-sulfonic acid is used as stabilizer. (+)-(1S)-camphor-10-sulfonic acid being a very effective resolving agent. . Chiral polyaniline(PANI) nanofibers were synthesized via facilely potentiostatic electropolymerization method without template in the presence of(1S)-(+)camphor-10-sulfonic acid(D-CSA) or(1R)-(-)camphor-10-sulfonic acid(L-CSA) as the dopant. Synthesis of QUATs derived from (1S)-(+)camphor-10-sulfonic acid. |
| Uses | Used as a resolving agent, and as a catalyst for coupling dipeptides. |
| Definition | ChEBI: (S)-camphorsulfonic acid is the S enantiomer of camphorsulfonic acid. It is a conjugate acid of a (S)-camphorsulfonate. It is an enantiomer of a (R)-camphorsulfonic acid. |
| Purification Methods | Crystallise the acid from ethyl acetate and dry it under vacuum (deliquescent). [Loudon J Chem Soc 823 1933, Komppa J Prakt Chem 162 19 1943, Beilstein 11 IV 642.] See above for RS-isomer. |
Product picture
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
