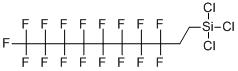
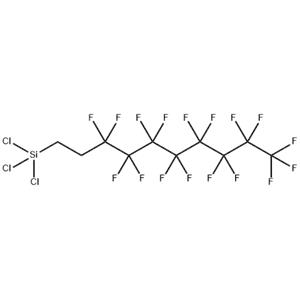
| Details: | Molecular formula: C10H4Cl3F17Si Molecular weight: 581.5
Appearance:colorless transparent liquid Boiling point (760mmHg): 90℃ Specific gravity (15℃) : 1.715g/ml
Chemical Properties:colourless to straw-coloured liquid with an odour of hydrogen chloride
Uses:1H,1H,2H,2H-Perfluorodecyltrichlorosilane, also known as FDTS, is a colorless liquid chemical with molecular formula C10H4Cl3F17Si. FDTS molecules form self-assembled monolayers. They bond onto surfaces terminated with hydroxyl (-OH) groups, such as glass, ceramics, or SiO2 forming a regular covalent bond. It anchors on oxide surfaces with its tricholoro-silane group and attaches covalently.
A 1H,1H,2H,2H-Perfluorodecyltrichlorosilane (FDTS) monolayer is often applied to movable microparts of microelectromechanical systems (MEMS). A FDTS monolayer reduces surface energy and prevents sticking, so they are used to coat micro- and nano-features on stamps for a nanoimprint lithography which is becoming a method of choice for making electronics, organic photodiodes, microfluidics and other. Reduced surface energy is helpful for reduction of ejection force and demolding of polymer parts in an injection molding and A 1H,1H,2H,2H-Perfluorodecyltrichlorosilane (FDTS) coating was applied onto some metallic injection molding molds and inserts. Further, it is used to coat micro-nano features on stamps for a nano imprint lithography. In addition, it is used in the preparation of other products by reacting with methanol.
1H,1H,2H,2H-Perfluorodecyltrichlorosilane (CAS# 78560-44-8) is a useful compound used as part of antireflective coatings for solar cell efficiency improvement. The high level of hydrophobicity of 1H,1H,2H,2H-Perfluorodecyltrichlorosilane also makes it useful as coating for electrical insulators.
DTS(FBTTh2)2 can be used as a conjugating polymer that forms a donor-acceptor system with acceptor molecules such as perylene diimide, PC71BM and other fullerenes for the fabrication of bulk-heterojunction based solar cells.
DTS(FBTTh2)2 is a conductive polymer that can be used as a donor molecule. It has a narrow band gap and shows a maximum power conversion efficiency of 7.0%. Its photostability is more than that of P3HT. |

 China
China