| Overview | Boron (B), the fifth element in the periodic chart, is ubiquitous in the environment, where it is found combined with O to form compounds called inorganic borates (e.g., borax). Natural sources of borates in the environment include soils, rocks, surface and ocean waters, and the atmosphere.
B in the form of borates has long been recognized as an essential plant micronutrient for the growth and viability of plants. Recently, there has been a growing body of evidence that B may be an essential element for frogs, fish, rats, and humans, as well as for plants[1-5]. The major sources of B exposure are diet and drinking water. Fruits, vegetables, and nuts are especially rich in B. Rainey et al.[6] recently studied daily dietary B intake, evaluating the food consumption records of over 25,000 Americans over several days. The median, mean, and 95 percentile B intake for all participants were 0.76, 0.93, and 2.4 mg B/d, respectively.
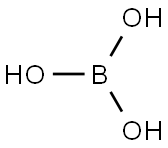
Boric acid (H3BO3) is a boron compound that is soluble and circulates in plasma[7]. It is a colorless, water-soluble, salt-like white powder, which have been used as pesticide since 1948. Normally, it is used to kill mites, insects, fungi and algae. For instances fleas, cockroaches, termites and wood decay fungi[8, 9]. Borate chemicals and boric acid have been used extensively for industrial purposes and its salts have been used for medication as an antiseptic to kill bacteria and fungi. Normally, it is used in the form of powder and liquid; depending to the target and conditions of pest, boric acid might applied as a spray or aerosol, as well as in the form of tablets, granule, pellets, paste or crystalline[10]. |
| Applications | Boric acid plays a role as a “stomach poison” for certain pest such as cockroaches, ants and termites. As an insecticide, it usually applied in bait form or used as a dry powder in which containing a feeding attractant and then added into crevices and creaks so that it forms a layer of dust[11]. So boric acid adheres to their legs when the insects move across the powder. Hence, they may ingest the poison when the insects groom themselves. This will causes death due to starvation and dehydration after 3-10 days[9]. However, the insecticide mechanism of boric acid on insects has not been satisfactorily developed. Some hypotheses has been suggested including death by starvation owing to abrasive effect on the cuticle then cause destruction or slow drying of foregut cells[12, 13].
Besides that, when boric acid used as an herbicide, it desiccates or disrupts the photosynthesis system in plants. Hence, boric acid is normally used to suppress algae in swimming pools and sewage systems[8]. On the other hand, as a fungicide, the fungicidal properties of boric acid prevent the production of conidia or asexual spores of the fungi; hence, it suppresses the growth of fungi. Therefore, boric acid is used as wood preservative in wood industry such as lumber and timber products that controls decay producing fungi[9,14].
Boric acid is reported to be used as food preservatives in some foods and food products. Boric acid is used for preserving meats, meat products, caviar and dairy products[15]. This is because boric acid is able to inhibit the growth of microorganism, therefore, the preserved food can stay fresh and longer[16]. Moreover, according to Yiu et al. (2008)[17], boric acid was added to some food products to control starch gelatinization, as well as enhance the color, texture and flavor of the food. |

 China
China