| CAS: | 504-02-9 |
| MF: | C6H8O2 |
| MW: | 112.13 |
| EINECS: | 207-980-0 |
| Product Categories: | ketone;Intermediates;Analytical Chemistry;Carbonyl Group Labeling Reagents for Fluorescence HPLC;Fluorescence Detection (HPLC Labeling Reagents);Isotope Labelled Compounds;cyclic compounds;HPLC Labeling Reagents;bc0001 |
| Mol File: | 504-02-9.mol |
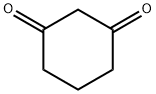 |
|
| 1,3-Cyclohexanedione Chemical Properties |
| Melting point | 101-105 °C (lit.) |
| Boiling point | 170.05°C (rough estimate) |
| density | 1,1 g/cm3 |
| vapor pressure | 0.018Pa at 25℃ |
| refractive index | 1.4576 (estimate) |
| storage temp. | Store at +2°C to +8°C. |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 5.26(at 25℃) |
| form | Powder |
| color | Pale yellow to beige |
| Water Solubility | soluble |
| Merck | 14,3178 |
| BRN | 385888 |
| LogP | 0.461 at 21.4℃ and pH3.2 |
| Surface tension | 69.9mN/m at 1.01g/L and 22℃ |
| CAS DataBase Reference | 504-02-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3-Cyclohexanedione(504-02-9) |
| EPA Substance Registry System | 1,3-Cyclohexanedione (504-02-9) |
| 1,3-Cyclohexanedione Usage And Synthesis |
| Chemical Properties | pale yellow to beige powder |
| Uses | 1,3-Cyclohexanedione is used in organic synthesis and pharmaceutical intermediates. It is the intermediate of herbicides sulcotrione and nitrosulfonone. It is also nitisinone intermediate. |
| Uses | 1,3-Cyclohexanedione can be used as a building block in the synthesis of:
9-substituted 1,8-dioxo-xanthenes by reacting with unactivated aldehydes via aldol condensation/ Michael addition reaction. [(1,2,3-triazol-4-yl)methoxy-phenyl]-2H-indazolo[2,1-b]phthalazine-trione derivatives by treating with phthalhydrazide, aromatic propargyloxy aldehydes, and azides via a four-component condensation reaction.
It can be also used to prepare 2-substituted adducts, which are important intermediates for the synthesis of ?-enaminones , polyhydroquinoline derivatives , 1,8-dioxo-dodecahydroxanthenes , spirocyclopentanols , oxathioles , and triquinanes. |
| Definition | ChEBI: 1,3-Cyclohexanedione is a cyclohexanedione carrying oxo substituents at positions 1 and 3. It is a beta-diketone and a cyclohexanedione. |
| Synthesis Reference(s) | The Journal of Organic Chemistry, 30, p. 3206, 1965 DOI: 10.1021/jo01020a506 |
| Purification Methods | Crystallise the dione from *benzene. Dissolve ~50g of the diol in 140mL of *C6H6 under N2, cool, collect the solid and dry it in a vacuum desiccator overnight. It is unstable and should be stored under N2 or Ar at ~0o. [Thompson Org Synth Coll Vol III 278 1955, Beilstein 7 IV 1985.] |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
