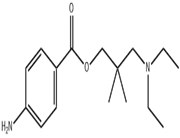
Chemical and physical properties:
Density: 1.0±0.1 g/cm3
Boiling point: 372.7±52.0°C at 760 mmHg
Melting point: 66-69°C
Molecular formula: C 14 H 22 N 2 O
Molecular weight: 234.337
Flash point: 179.2±30.7°C
Accurate quality: 234.173218
Logarithm: 3.63
Steam pressure: 0.0±0.9 mmHg at 25°C
Refractive index: 1.512
Storage situation: stored at RT
Stable: stable. Incompatible with strong oxidants.
Water solubility: almost insoluble
use:
Lidocaine amide local anesthetics have anti-inflammatory effects in vivo and in vitro, which may be due to the reduction of pro-inflammatory cytokines, intracellular adhesion molecule 1 (ICAM-1) and reduced neutrophil influx. Common local anesthesia and antiarrhythmic drugs. Lidocaine can be used locally to relieve itching, burning and pain caused by dental inflammation. It can be injected as a dental anesthetic or local anesthetic for minor operations. Lidocaine is the first aminoamide local anesthetic, which was first synthesized by Swedish chemist Nils Lofgren in 1943 under the name of xylidine. His colleague Bengt Lundqvist personally conducted the first injection anesthesia experiment. Lidocaine is metabolized (dealkylated) by CYP3A4 in the liver by approximately 95%, becomes the pharmacologically active metabolite monoethylglycoside (MEGX), and is then metabolized by the inactive glycine xylidine. MEGX has a longer half-life than lidocaine, but sodium channel blockers are less effective. In most patients, the elimination half-life of lidocaine is about 90-120 minutes. Patients with liver insufficiency (average 343 minutes) or congestive heart failure (average 136 minutes) may extend this time
Biological activity:
Lidocaine is an amide local anesthetic, which has anti-inflammatory effects in vivo and in vitro, which may be caused by the decrease of pro-inflammatory cytokines, intracellular adhesion molecule 1 (ICAM-1) and decreased neutrophil influx . Common local anesthesia and antiarrhythmic drugs. Lidocaine can be used locally to relieve itching, burning and pain caused by dental inflammation. It can be injected as a dental anesthetic or local anesthetic for minor operations. Lidocaine is the first aminoamide local anesthetic, which was first synthesized by Swedish chemist Nils Lofgren in 1943 under the name of xylidine. His colleague Bengt Lundqvist personally conducted the first injection anesthesia experiment. Lidocaine is metabolized (dealkylated) by CYP3A4 in the liver by approximately 95%, becomes the pharmacologically active metabolite monoethylglycoside (MEGX), and is then metabolized by the inactive glycine xylidine. MEGX has a longer half-life than lidocaine, but sodium channel blockers are less effective. In most patients, the elimination half-life of lidocaine is about 90-120 minutes. Patients with liver insufficiency (average 343 minutes) or congestive heart failure (average 136 minutes) may extend this time.
Welcome to contact us and look forward to cooperating with you!!!
Contact details:
Whatsapp / Skype / WeChat: +8613373414058
Email: gantuo02@cngantuo.com




 China
China