| CAS: | 3355-28-0 |
| MF: | C4H5Br |
| MW: | 132.99 |
| EINECS: | 608-891-3 |
| Product Categories: | Intermediates;Acetylenes;Functionalized Acetylenes;Alkynyl;Halogenated Hydrocarbons;Organic Building Blocks |
| Mol File: | 3355-28-0.mol |
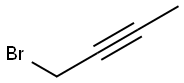 |
|
| 1-BROMO-2-BUTYNE Chemical Properties |
| Boiling point | 40-41 °C/20 mmHg (lit.) |
| density | 1.519 g/mL at 25 °C (lit.) |
| refractive index | n20/D 1.508(lit.) |
| Fp | 97 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Miscible with acetonitrile. |
| form | Liquid |
| Specific Gravity | 1.519 |
| color | Clear pale yellow-greenish |
| BRN | 605306 |
| InChIKey | LNNXOEHOXSYWLD-UHFFFAOYSA-N |
| CAS DataBase Reference | 3355-28-0(CAS DataBase Reference) |
| 1-BROMO-2-BUTYNE Usage And Synthesis |
| Chemical Properties | Clear pale yellow-greenish liquid |
| Uses | 1-Bromo-2-butyne is used in the preparation of six to eight annulated ring compounds in reaction with indoles and pseudopterane (+/-)-Kallolide B, which is a marine natural product. Further, it acts as a precursor in the preparation of axially chiral teranyl compounds, alkylation of L-tryptophan methyl ester, 4-butynyloxybenzene sulfonyl chloride and mono-propargylated diene derivative. In addition to this, it is also used in the synthesis of isopropylbut-2-ynylamine, allenylcyclobutanol derivatives, allyl-[4-(but-2-ynyloxy)phenyl]sulfane, allenylindium and axially chiral teranyl compounds. |
| Preparation | 1-bromo-2-butyne is obtained by reacting 2-butyn-1-ol and organic solvent, pyridine.First react bromoethane with metal magnesium in THF to prepare Grignard reagent, then add paraformaldehyde to Grignard reagent for Grignard reaction, separate and recover after Grignard reaction 2-butyn-1-ol, then mix 2-butyn-1-ol with organic solvent and pyridine, add phosphorus tribromide dropwise for bromination reaction, separate and recover the product after bromination reaction. |
| General Description | 1-Bromo-2-butyne is a propargyl bromide derivative. It is one of the constitutional isomer of bromo butyne. Its Br-loss threshold photoionization breakdown diagram has been analyzed to derive dissociative photoionization thresholds to C4H5+ production. It participates in the preparation of linagliptin. |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
