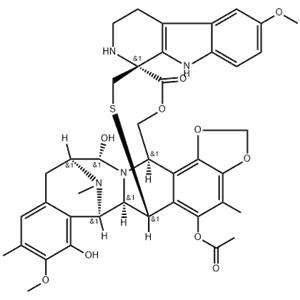
| Description | Lurbinectedin (PM01183) is a new DNA minor groove covalent binder with potent anti-tumour activity; inhibits RMG1 and RMG2 cell growth with IC50 values of 1.25 and 1.16 nM, respectively. |
|---|
| Related Catalog | Signaling Pathways >> Others >> Others Research Areas >> Cancer |
|---|
| Target | IC50: 1.25 nM (RMG1), 1.16 nM (RMG2)[1] |
|---|
| In Vitro | PM01183 is a new synthetic tetrahydroisoquinoline alkaloid that is currently in phase I clinical development for the treatment of solid tumours. PM01183–DNA adducts in living cells give rise to double-strand breaks, triggering S-phase accumulation and apoptosis. The potent cytotoxic activity of PM01183 is ascertained in a 23-cell line panel with a mean GI50 value of 2.7 nM[2]. Lurbinectedin exhibits significant antitumor activity toward chemosensitive and chemoresistant human ovarian clear cell carcinoma (CCC) cells in vitro[1]. |
|---|
| In Vivo | Mouse CCC cell xenografts reveals that lurbinectedin significantly inhibits tumor growth. Lurbinectedin plus SN-38 results in a significant synergistic effect[1]. In four murine xenograft models of human cancer, PM01183 inhibits tumour growth significantly with no weight loss of treated animals[2]. Single lurbinectedin or cisplatin-combined therapies are effective in treating cisplatin-sensitive and cisplatin-resistant preclinical ovarian tumor models. The strongest synergistic effect is observed for combined treatments, especially in cisplatin-resistant tumors. Lurbinectedin tumor growth inhibition is associated with reduced proliferation, increased rate of aberrant mitosis, and subsequent induced apoptosis[3]. |
|---|
| Cell Assay | The MTS assay is used to analyze the effects of each drug. Cells are plated in 96-well plates and treated with PM01183 (0, 0.1, 0.3, 1, 3 nM) . After 48 hours’ incubation, the number of surviving cells is assessed by determining the A490nm of the dissolved formazan product after the addition of MTS for 1 hour. Cell viability is calculated as follows: Aexp group / Acontrol×100[1]. |
|---|
| Animal Admin | Mice: Mice are transplanted with fragments of OVAX1 and OVAX1R tumors, and when tumors reaches a homogeneous palpable size are randomly allocated into the treatment groups: i) Placebo; ii) Lurbinectedin (0.18 mg/kg); iii) Cisplatin (3.5 mg/kg); and iv) Lurbinectedin plus cisplatin (0.18 + 3.5 mg/kg). Drugs are i.v. administered once per week for 3 consecutive weeks (days 0, 7, and 14). Seven days after the final dose (day 21), animals are sacrificed, their ovaries dissected out, and weighed. Representative fragments are either frozen in nitrogen or fixed and then processed for paraffin embedding[3]. |
|---|
| References | [1]. Takahashi R, et al. Preclinical Investigations of PM01183 (Lurbinectedin) as a Single Agent or in Combination with Other Anticancer Agents for Clear Cell Carcinoma of the Ovary. PLoS One. 2016 Mar 17;11(3):e0151050. [2]. Leal JF, et al. PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Br J Pharmacol. 2010 Nov;161(5):1099-110. [3]. Vidal A, et al. Lurbinectedin (PM01183), a new DNA minor groove binder, inhibits growth of orthotopic primary graft of cisplatin-resistant epithelial ovarian cancer. Clin Cancer Res. 2012 Oct 1;18(19):5399-411. |
|---|

 China
China