1/2
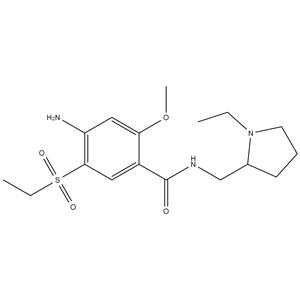
Amisulpride NEW
- Min. Order1KG
- Purity99%
- Cas No71675-85-9
- Supply Abilityg-kg-tons, free sample is available
- Update time2024-04-16

| Product Name | Amisulpride |
| CAS No | 71675-85-9 |
| EC-No | |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | g-kg-tons, free sample is available |
| Release date | 2024/04/16 |
1. Materials information
Names
| Name | amisulpride |
|---|---|
| Synonym | More Synonyms |
Amisulpride Biological Activity
| Description | Amisulpride is a dopamine D2/D3 receptor antagonist with Kis of 2.8 and 3.2 nM for human dopamine D2 and D3, respectively. |
|---|---|
| Related Catalog | Signaling Pathways >> GPCR/G Protein >> Dopamine Receptor Signaling Pathways >> Neuronal Signaling >> Dopamine Receptor Research Areas >> Neurological Disease Research Areas >> Cancer |
| Target | Ki: 2.8 nM (D2 receptor), 3.2 nM (D3 receptor)[1] |
| In Vitro | Amisulpride is an atypical dopamine D2/D3 receptor antagonist with Kis of 2.8 and 3.2 nM for human dopamine D2 and D3, respectively. Amisulpride (100 nM) inhibits quinpirole-elicited [3H]thymidine incorporation with an IC50 value of 22±3 nM (n=3). Amisulpride slightly but significantly increases [3H]dopamine release from slices of the rat striatum (S2/S1=0.88±0.04 under control conditions, n=6; 1.04±0.08 in the presence of 100 nM Amisulpride,n=4; P<0.05) and opposes the inhibitory effects of 7-OH-DPAT in both brain areas[1]. |
| In Vivo | Only the highest dose of Amisulpride (100 mg/kg) significantly reduces dopamine levels in the striatum or limbic system. Amisulpride significantly increases the synthesis of dopamine in the rat striatum and limbic system at doses of 20 and 100 mg/kg. Amisulpride (0.5 to 75 mg/kg) fails to provoke an additional increase in dopa accumulation in the striatum but slightly accelerates, at 75 mg/kg, dopamine synthesis in the limbic system. In comparison with vehicle-treated controls, Amisulpride (10 mg/kg) increases extracellular dopamine levels. The administration of Amisulpride (0.5 to 15 mg/kg s.c.) provokes a time- and dose-dependent increase in the stimulation-evoked dopamine release. Amisulpride decreases striatal ACh levels significantly at 30 and 100 mg/kg (87.5% and 56.3% of control levels, respectively)[1]. In both acute study, Amisulpride (70 mg/kg, p.o.) significantly increases the duration of swimming behavior [F(3,28)=45.90, p<0.01][2]. |
| Cell Assay | The functional effects of Amisulpride at the dopamine D3 receptor subtype are assessed. Briefly, the mitogenic response elicited in NG108-15 neuroblastoma-glioma cells stably transfected with human dopamine D3 receptor cDNA by the addition of 10 nM quinpirole in the presence of 1 μM forskolin is quantified by the incorporation of [3H]thymidine. Antagonism of quinpirole-induced mitogenesis is measured in the presence of increasing (0.1 to 100 nM) concentrations of Amisulpride[1]. |
| Animal Admin | A total of 64 male Swiss albino mice weighing between 20 to 30 g are used. The animals are fed with standard pellet diet and water ad libitum. The mice are divided in different groups (n=8 in each group) and drug administration is done as follows: Group 1 (control): distilled water (1 mL/kg) 23.5, 5 and 1 h before the test. Group 3 (Amisulpride): Amisulpride (70 mg/kg) 23.5, 5 and 1 h before the test[2]. |
| References | [1]. Schoemaker H, et al. Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. J Pharmacol Exp Ther. 1997 Jan;280(1):83-97. [2]. Pawar GR, et al. Evaluation of antidepressant like property of amisulpride per se and its comparison with fluoxetine and olanzapine using forced swimming test in albino mice. Acta Pol Pharm. 2009 May-Jun;66(3):327-31. |
Chemical & Physical Properties
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 558.9±50.0 °C at 760 mmHg |
| Melting Point | 124-128ºC |
| Molecular Formula | C17H27N3O4S |
| Molecular Weight | 369.479 |
| Flash Point | 291.8±30.1 °C |
| Exact Mass | 369.172241 |
| PSA | 110.11000 |
| LogP | 1.60 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.546 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ≥5 mg/mL |
MSDS
Amisulpride MSDS(Chinese) |
Toxicological Information
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
Safety Information
| Symbol |  GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CV2308701 |
| HS Code | 2933990090 |
Synthetic Route
Previous 1/3 Next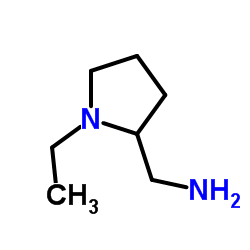 2-(Aminomethyl)... CAS#:26116-12-1 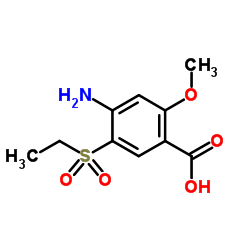 4-Amino-5-(ethy... CAS#:71675-87-1 ~70% 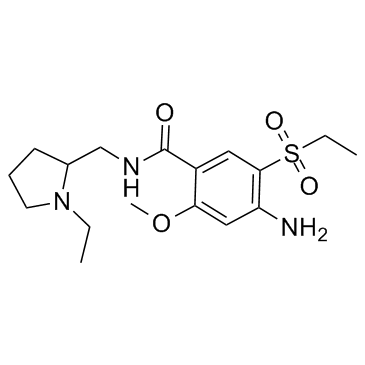 Amisulpride CAS#:71675-85-9 |
| Literature: LUPIN LIMITED; PAGHDAR, Dinesh, Jayntibhai; KOLEKAR, Mahesh, Ramkumar; DESHPANDE, Tushar, Nandkumar; PATIL, Suryaprakash, Pandurang; CHAVAN, Yuvraj, Atmaram; RAY, Purna, Chandra; SINGH, Girij, Pal Patent: WO2011/158084 A1, 2011 ; Location in patent: Page/Page column 14-15 ; |
 2-(Aminomethyl)... CAS#:26116-12-1 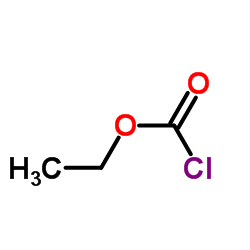 Ethyl chloroformate CAS#:541-41-3 ~99%  Amisulpride CAS#:71675-85-9 |
| Literature: Societe d'Etudes Scientifiques et Industrielles de l'Ile de-France Patent: US4294828 A1, 1981 ; |
 2-(Aminomethyl)... CAS#:26116-12-1  4-Amino-5-(ethy... CAS#:71675-87-1  Ethyl chloroformate CAS#:541-41-3 ~61%  Amisulpride CAS#:71675-85-9 |
| Literature: Societe d'Etudes Scientifiques et Industrielles de l'Ile de-France Patent: US4294828 A1, 1981 ; |
Precursor & DownStream
| Precursor 7 | Previous 1/2 Next |
|---|---|
| |
| DownStream 0 | |
Customs
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
Articles2
More Articles| The antipsychotic amisulpride: ultrastructural evidence of its secretory activity in salivary glands. Oral Dis. 20(8) , 796-802, (2014) Amisulpride is reported to inhibit clozapine-induced sialorrhea. Preclinically, clozapine evokes muscarinic-M1-type-mediated secretion that, however, amisulpride does not reduce. Instead, amisulpride,... | |
| A double-blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. Int. Clin. Psychopharmacol. 19 , 63-69, (2004) Atypical antipsychotics offer advantages over earlier drugs for the treatment of schizophrenia, although few data exist on the relative merits of different atypical antipsychotics. A multicentre, doub... |
Synonyms
| Amisulpiride |
| Aminosultopride |
| Socian |
| Benzamide, 4-amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxy- |
| 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide |
| Amisulpride |
| 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamide |
| Amisulpridum [INN-Latin] |
| amisulpridum |
| 4-amino-N-[(1-ethyl-2-pyrrolidin-yl)methyl]-5-(ethyl-sulfonyl)-2--methoxybenzamide |
| MFCD00866691 |
| Deniban |
| EINECS 275-831-7 |
| Solian |
| UNII:8110R61I4U |
| 4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-o-anisamide |
| amisulprida |
| 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide |
2. Packaging of materials
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping & Delivery
By Express
Provide door to door service
Suitable for goods under 50kg
Delivery: 3-7 days
Cost: low cost

By Air
Provide airport to airport service
Suitable for goods over 50kg
Delivery: 3-14 days
Cost: high cost

By Sea
Provide seaport to seaport service
Suitable for goods over 100kg
Delivery: 2-45 days
Cost: low cost

4. Contact information
For more details, pls contact us freely.
Email address: Tina@fdachem.com
Mob: 86 13213167925
WhatsApp/Skype/Wechat/LINE: 86 13213167925
Company Profile Introduction
Henan Fengda Chemical Co., Ltd. is located in the High-tech Development Zone of Henan Province. Specializing in the production and sales of various fine chemical products required for industrial production, including chemical raw materials, organic raw materials, petrochemicals, chemical reagents, solvents, catalysts, and additives, etc.

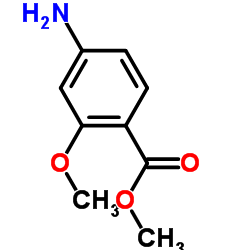 CAS#:27492-84-8
CAS#:27492-84-8

