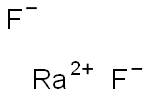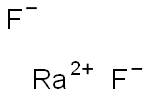This compound has the chemical formula of RaF2 and
a molecular weight of 263.8214 g/mol. Its structure is
similar to that of CaF2, the cubic fluoride. It can be
prepared by reaction of HF gas on the metal:
Ra+ 2HF→RaF2+H2
Although this compound is listed as a viable salt of
radium, no scientific studies of the properties of this
material are readily available. Furthermore, RaF2 is not
offered for sale nor are there any descriptions of its physical and chemical properties. However, this compound has been given a CAS number of 20610-49-5, presumably
because of its dangerous inherent radioactivity.