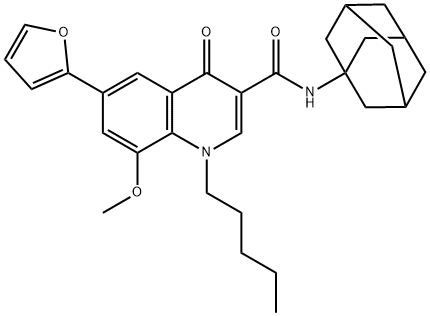
ki: 8.5 nm4-quinolone-3-carboxamide furan cb2 agonist is a high-affinity ligand of cb2.the endocannabinoid system consists of endogenous cannabinoids (endocannabinoids), cannabinoid receptors (primarily cb1 and cb2), and the enzymes that synthesize and degrade endocannabinoids.
previous study found that 4-quinolone-3-carboxamide furan cb2 agonist (4g) was devoid of any potential “indirect” agonist activity at cannabinoid receptors, exerted by prolonging the lifespan of endocannabinoids because 4g at up to a 10 μm concentration did not inhibit anandamide or 2-ag degradation by faah or magl, respectively. in cytotosicity study, 4g was tested at 1 μm and the results showed that it exhibited very low or no cytotoxicity, the cell viability being above 95% after a 72 h treatment [1].
in animal study, 4g was found to have antinociceptive activity in the formalin test in mice. moreover, 4g was very potent with maximal effect being reached at the 1 mg/kg dose and efficacious also on the first phase of the nocifensive response. the effect of 4g could be strongly reduced by the addition of am630, a cb2-selective antagonist/inverse agonist, therefore demonstrating that 4g might act as a potent and selective cb2 agonist [1].
[1] s. pasquini, m. de rosa, v. pedani, et al. investigations on the 4-quinolone-3-carboxylic acid motif. 4. identification of new potent and selective ligands for the cannabinoid type 2 receptor with diverse substitution patterns and antihyperalgesic effects in mice. journal of medicinal chemistry 54, 5444-5453 (2011).