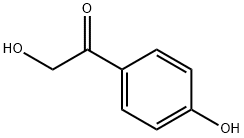
Preparation by reaction of p-hydroxy-a-bromoacetophenone with formic acid in the presence of DBU,
in benzene at 0°, followed by saponification of the intermediate formate ester with sodium hydroxide in methanol (99%)
in methylene chloride, with the same treatment (49%).
ChEBI: 2,4'-dihydroxyacetophenone is a dihydroxyacetophenone that is ethanone substituted by a hydroxy group at position 2 and a 4-hydroxyphenyl group at position 1. It is a member of phenols, a primary alpha-hydroxy ketone and a dihydroxyacetophenone.