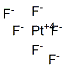Dark-red octahedral crystals; volatile and unstable; density 3.83g/cm3; melts at 61.3°C; vaporizes at 69.14°C; reacts violently with water.
Platinum hexafluoride does not have many commercial applications. It is used as a strong oxidizing agent and can oxidize oxygen from the air. It is used in research. Platinum hexafluoride forms compounds with molecular oxygen and xenon, [O2+][PtF6 –] and XePtF6 , respectively.
The hexafluoride is a very powerful oxidizing agent reacting violently with most oxidizable substances. Reaction with liquid water is violent forming HF, oxygen, lower fluorides of platinum, and other products. In vapor phase hydrolysis occurs more smoothly.
The hexafluoride decomposes on heating; also decomposed by UV radiation to lower fluorides; and reacts with the inert gas xenon, forming a solid product, Xe(PtF6). It reacts with molecular oxygen to produce O2+PtF6 – The compound attacks glass at ordinary temperatures.
Platinum hexafluoride is dangerously corrosive. Inhalation of its vapors or skin contact causes serious injury. Also, it can react explosively with a number of substances.
dark brick red, rhomb solid; strong oxidizing agent; there are also PtF4, 13455-15-7, and PtF5, 13782-84-8 [KIR82]