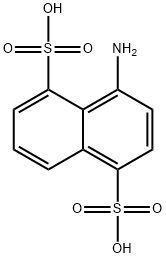
1-Aminonaphthalene-4,8-disulfonic acid [117-55-5]. (4-aminonaphthalene-1,5- disulfonic acid), C10H9NO6S2, Mr 303.3, and its acid monosodium salt are sparingly soluble in cold water, but the disodium salt is readily soluble. Hydrolysis with sodium hydroxide at 200 ℃ yields 1-amino-8-hydroxynaphthalene-4-sulfonic acid; at higher temperature, 1,8-dihydroxynaphthalene-4-sulfonic acid is formed. Heating with bisulfite produces 1-hydroxynaphthalene4,8-disulfonic acid. Diazo compounds couple with 1-aminonaphthalene-4,8-disulfonic acid to form azo compounds, in contrast to 2-aminonaphthalene-4,8-disulfonic acid, which reacts to form diazoamino compounds.
Naphthalene is sulfonated under conditions that give predominantly the 1,5- disulfonic acid, and the sulfonation mass is nitrated at 20 ℃. 3-Nitronaphthalene-1,5-disulfonic acid is precipitated from the quenched reaction mixture as its iron(II) salt and removed. After dilution and liming out, the remainder of the mixed nitro solution is reduced with iron and dilute acid, and the 1,4,8-isomer is salted out preferentially in ca. 40 % yield. A small proportion (ca. 10 % of 1-aminonaphthalene-3,8-disulfonic acid) is then salted out, after which the more soluble 1-aminonaphthalene-5,7-disulfonic acid crystallizes on concentration.