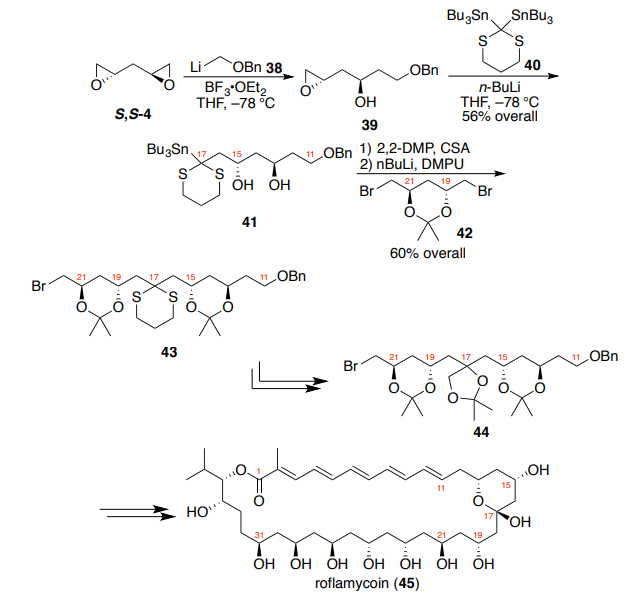
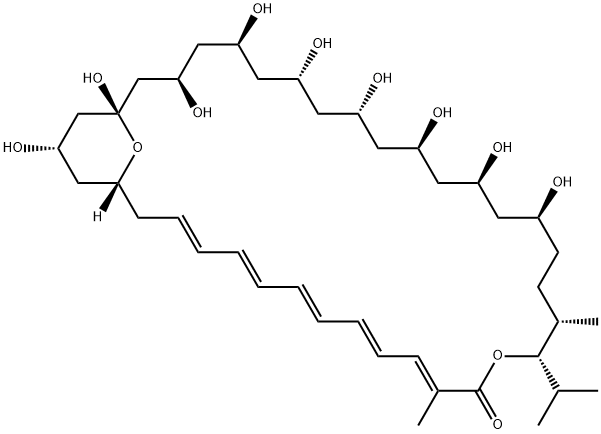
For Rychnovsky and co-worker’s synthesis of roflamycoin (45), the
hemiketal-pyran linkage required differential substitution (Scheme 9) and
demonstrated the power of the sequential double addition strategy.14
Treatment of bis-epoxide S,S-4 with a stoichiometric amount of
(benzyloxy)methyllithium 38 and BF3?OEt2 in THF at –78 °C provided monoaddition adduct 39, which was then treated with the organolithium formed
from transmetallation of 2,2-bis-(tributyltin)dithiane 40. This single-pot
procedure afforded dithiane diol 41 in 56% overall yield. Subsequent
acetonide formation and transmetallation provided a competent nucleophile
for direct displacement of dibromide 42, which itself was derived from
dichlorodiol R,R-3. The resulting bis-acetonide (43) was obtained in 60%
overall yield and provided a rapid access to tris-acetonide 44, a key
intermediate for the synthesis of the hemiketal-pyran moiety of 45. Similar
sequential addition strategies have proven to be useful as well.