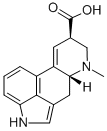
A precursor of the semisynthetic ergot
derivatives, but having no biological activity itself. It is subject
to controls under the Controlled Substances Act of 1970 in the
United States, since it is the immediate precursor for the synthesis
of LSD. Lysergic acid is obtained by hydrolysis of ergot
alkaloids, either obtained from grains infected with Claviceps
or, more commonly, by fermentation in submerged culture.
Labelled Lysergic acid. Lysergic acid and iso-lysergic acid are the main cleavage products formed on alkaline hydrolysis of the alkaloids which are characteristic of ergot. Potent hallucinogen; non-selective serotonin receptor agonist.
Controlled substanc
Lysergic acid and iso-lysergic acid are the main cleavage products formed on alkaline hydrolysis of the alkaloids which are characteristic of ergot. Potent hallucinogen; non-selective serotonin receptor agonist.
Controlled substance.
ChEBI: An ergoline alkaloid comprising 6-methylergoline having additional unsaturation at the 9,10-position and a carboxy group at the 8-position.
Flammability and Explosibility
Not classified
It crystallises from water as a hydrate. The methyl ester crystallises from C6H6 and has m 168o; the amide [478-94-4] has m 242o(dec) (from MeOH) and []546 +15o (c 0.5, pyridine).The (-)-hydrochloride has m 208-210o(dec, from MeOH). [Kornfeld et al. J Am Chem Soc 7 6 5256 1954,Kornfeld et al. J Am Chem Soc 78 3087 1956, Beilstein 25 III/IV 934,]